Material-independent fabrication of superhydrophobic surfaces by mussel-inspired polydopamine†
Abstract
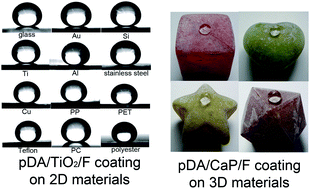
This study reports methods for general preparation of superhydrophobic surfaces on any type of material surface using mussel-inspired poly(dopamine) (pDA). The use of pDA presents several advantages over conventional superhydrophobic fabrication methods: development of superhydrophobicity in a material-independent manner, enhancement of mechanical stability, decreases in angle hysteresis, and applicability to 3D objects.

 Please wait while we load your content...
Please wait while we load your content...