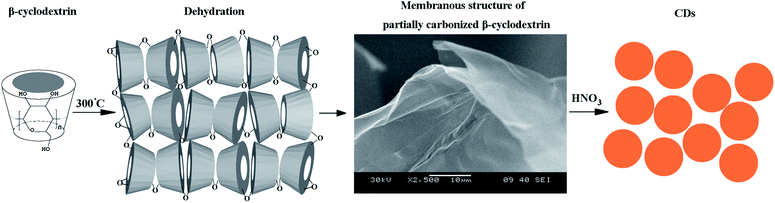
High-quality carbon dots: synthesis, peroxidase-like activity and their application in the detection of H2O2, Ag+ and Fe3+
Wengfeng
Zhu
,
Jian
Zhang
,
Zhenchao
Jiang
,
Weiwei
Wang
and
Xiaoheng
Liu
*
Key Laboratory for Soft Chemistry and Functional Materials of Ministry Education, Nanjing University of Science and Technology, Nanjing 210094, China. E-mail: xhliu@mail.njust.edu.cn; Fax: +86-25-8443-2747; Tel: +86-25-8431-5943
First published on 19th February 2014
Abstract
In this paper, bright and high-quality carbon dots (CDs) were prepared by refluxing a membranous carbonized β-cyclodextrin in nitric acid. The structure and optical properties of the CDs were characterized by scanning electron microscopy (SEM), transmission electron microscopy (TEM), Fourier transform infrared spectroscopy (FT-IR), fluorescence spectrophotometry (FL), X-ray diffraction (XRD) and Raman spectroscopy. The mechanism for the formation of the CDs is also discussed. As-prepared CDs could be used as peroxidase mimics for the detection of H2O2. The results indicate that the CDs are capable of rapid and sensitive detection of H2O2 in the linear range from 2 × 10−6 to 5 × 10−4 mol L−1 and the limit of detection of H2O2 is as low as 1 × 10−6 mol L−1. More importantly, the peroxidase-like activity of the CDs is expanded to the detection of some oxidative ions (such as Fe3+ and Ag+). The linear ranges for Fe3+ and Ag+ were 8 × 10−6 to 1 × 10−4 mol L−1 and 5 × 10−6 to 1 × 10−4 mol L−1 respectively, with corresponding detection limits of 8 × 10−7 mol L−1 and 5 × 10−7 mol L−1 respectively.
1. Introduction
Since the discovery of carbon dots (CDs) by Xu et al. in 2004, a great deal of attention has been paid to improving the synthesis of the CDs, and significant breakthroughs have taken place.1 Generally, the synthesis routes are classified into two types: top-down and bottom-up methods. Top-down methods, such as arc discharge, laser ablation, electrochemical/chemical oxidation, usually involve expensive equipment and extreme conditions.2–8 So, bottom-up methods for preparing CDs from carbohydrates, L-ascorbic acid, candle soot, active carbon, EDTA, chitosan, and even egg white and orange juice, have been of great interest in recent years, and many synthetic methods have been developed, such as combustion, thermal/hydrothermal and ultrasonic/microwave methods.9–20 Bottom-up methods usually need tedious, time-consuming procedures, or complicated purification processes, which limit the development and application of CDs. Besides, the emission wavelengths of as-prepared CDs are generally monotonous with low yield. In this context, a cheap and fast synthesis route to give CDs in high yield is necessary for the large scale application of CDs.β-Cyclodextrin is a cyclic oligomer of seven units of α-D-glucose connected through glycosidic α-1,4 bonds.21 Due to its unique properties, β-cyclodextrin is frequently used as a building block for the construction of supramolecular complexes and has attracted much attention in the areas of drug formulation, analytical chemistry, agriculture and cosmetics.22 Novel structures are formed, giving a conical cylinder structure with a hydrophobic cavity and hydrophilic rim.23 In addition, β-cyclodextrin is also an excellent carbon source after carbonization.
As a new member of the carbon nanomaterial family, CDs are generally composed of sp2 hybridized carbon atoms with abundant oxygen- and hydrogen-containing residues with sizes below 10 nm.24–26 Compared with the organic fluorescent dyes and conventional metal-based quantum dots (QDs), CDs have many advantages, such as photostability, low toxicity and resistance to environmental change, thus, the applications of CDs have also been a subject of intense interest. CDs have already found potential applications in bioimaging, photocatalysis, light-emitting devices, optoelectronics and photocatalysis.16,26,27 Recently, Huang and coworkers reported that CDs could serve as peroxidase mimetics which could open up a new field for CD applications.28 As inorganic enzyme mimics, CDs possess some obvious advantages, such as their resistance towards inhibition or digestion by proteases.
In this paper, we proposed a simple route for the fabrication of bright and high-quality photoluminescent carbon dots, using β-cyclodextrin as a starting material. We discovered that carbonized β-cyclodextrins gave a membranous structure which proved to be a decisive factor in the preparation of CDs. The as-prepared CDs were shown to possess similar properties to peroxidase. The CDs could catalyze the oxidation reaction of peroxidase substrates in the presence of H2O2 and generated a blue solution. This unique property of the CDs was utilized for the fast and sensitive chromogenic detection of H2O2. We also extended the system to the detection of Fe3+ and Ag+. The results prove that the target concentration is proportional to the absorbance within a suitable range and the detection limit is very low.
2. Materials and methods
2.1. Materials
β-Cyclodextrin (C42H70O35, 98%), nitric acid (HNO3, 65–68%), anhydrous sodium carbonate (Na2CO3), acetic acid (C2H4O2, AR), sodium acetate trihydrate (CH3COONa·3H2O, AR), starch (C12H22O11, AR), hydrogen chloride (HCl, 37%) and sodium hydroxide (NaOH, AR) were purchased from Sinopharm Chemical Reagent Co., Ltd. 3,3′,5,5′-Tetramethylbenzidine (TMB, 98%) and peroxidase from horseradish (HRP, 100 units per mg) were obtained from Aladdin. All aqueous solutions were made using Milli-Q water.2.2. The synthesis and purification of the CDs
β-Cyclodextrin was placed in a tube furnace, and calcined at 300 °C for 2 h at a heating rate of 10 °C min−1 in air. The resulting black-foamed carbon residue possessed a metallic luster and was ground for further use.Typically, 2 g of carbonized β-cyclodextrin carbon residue was mixed with 50 mL of a 5 M nitric acid solution and refluxed for 12 h with stirring. The resulting dark brown solution was neutralized by Na2CO3.
Purified CDs were obtained by dialysis in water and dried at 60 °C in a vacuum oven. The obtained brown powder was redispersed in water to form 10 μg mL−1 solutions which were used for the following reactions.
2.3. Characterizations
The morphologies of the CDs and nanocomposites were characterized with a JEM-2100 transmission electron microscope (Jeol Ltd, Japan), using an accelerating voltage of 200 kV. SEM images were obtained with a JSE-6400F scanning electron microscope (Jeol Ltd, Japan), using an accelerating voltage of 30 kV. Fluorescence measurements were performed using a FL3-TCSPC spectrofluorometer (Horiba Jobin Yvon, France) equipped with a xenon arc lamp as the light source and a quartz cell (1 × 1 cm). UV-Vis absorption spectra were recorded with a 1201 spectrophotometer (Shimadzu, Japan) using a quartz cell with a 1 cm light path. The pH values were measured with a SG2 pH meter (Mettler Toledo, Shanghai, China). X-ray diffraction patterns of the QDs were recorded using a D8 ADVANCE X-ray powder diffractometer (Bruker, Germany) with Cu-Kα radiation (40 kV, λ = 1.54060 Å). IR spectra were collected using a VECTOR22 Fourier-transform infrared spectrometer (Bruker, Germany) in the range 4000–400 cm−1.2.4. Reactivity tests
Reactivity tests were performed as follows: 200 μL of H2O2 (or Fe3+, Ag+) with different concentrations and 70 μL of a 10 μg mL−1 CD solution, as well as 330 μL of TMB in a 0.5 M acetate buffer (pH 4.5) were added to 400 μL of a 0.5 M acetate buffer (pH 4.0). All the reaction solutions were firstly saturated with N2 to prevent the influence of dissolved oxygen. After being placed in a water bath at 45 °C for 5 min, the resulting solution was used for adsorption spectroscopy measurements. As for the detection of Fe3+ and Ag+, the mixture was irradiated with a 500 W xenon lamp and the resulting solution was used for adsorption spectroscopy measurements.In a control experiment, 10 ng mL−1 HRP in pH 4.0 acetate buffer was used instead of the CD solutions while keeping the other conditions the same.
3. Results and discussion
3.1. Characterizations
Fig. 1(A) shows typical infrared (IR) spectra of carbonized β-cyclodextrin (a) and CDs (b). In the IR spectrum for carbonized β-cyclodextrin, the typical –OH stretch peak does not appear around 3400 cm−1. The peaks at 1400 and 1084 cm−1 correspond to the asymmetric and symmetric stretching vibrations of C–O–C, respectively.15 The results indicate that β-cyclodextrin dehydrated and carbonized during the calcination process, which will be discussed in the reaction mechanism. In the IR spectrum of the CDs, the peak at 3422 cm−1 corresponds to the –OH stretching mode while the peak at around 1613 cm−1 corresponds to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching of the carbon skeleton of the CDs.10 The peaks at about 1675, 1235, and 1084 cm−1 are due to –COO−, implying the existence of large numbers of residual carboxyl groups.15 The abundance of –OH or –COOH groups covalently bonded to the carbon frameworks would improve the hydrophilicity and stability of the nanoparticles in aqueous solution. Thus, the application of CDs is greatly widened in biochemistry, diagnostics, drug delivery and so on.
C stretching of the carbon skeleton of the CDs.10 The peaks at about 1675, 1235, and 1084 cm−1 are due to –COO−, implying the existence of large numbers of residual carboxyl groups.15 The abundance of –OH or –COOH groups covalently bonded to the carbon frameworks would improve the hydrophilicity and stability of the nanoparticles in aqueous solution. Thus, the application of CDs is greatly widened in biochemistry, diagnostics, drug delivery and so on.
 | ||
| Fig. 1 (A) FTIR spectra of carbonized β-cyclodextrin (a) and CDs (b). (B) Raman spectrum of CDs prepared from carbonized β-cyclodextrin. | ||
Raman scattering was performed as shown in Fig. 1(B). The peaks at 1356 cm−1 were assigned to the D band, which is ascribed to the benzene ring-breathing vibrations, indicating the amorphous structure of the CDs, and the peak at 1578 cm−1 was assigned to the 1st order G band, which is related to the disordered sp2-bonded graphitic carbon. Also, the broad 1st G band (>100 cm−1, full width at half height) demonstrates that the CDs contain aromatic and olefinic molecules inside the particles.29
To gain insight into the structure of the CDs, TEM images of the CDs were obtained. As revealed in Fig. 2(A), the CDs are monodisperse spherical nanoparticles with uniform shape. Fig. 2(B) shows the corresponding size distribution of 5.45 ± 1.85 nm. Fig. 2(C) shows the HRTEM image of the CDs which clearly reveal that the CDs are monodispersed nanoparticles without crystallinity. XRD analysis was also conducted as shown in Fig. 2(D). The broad peak near 20° with a relatively low intensity is attributed to amorphous carbon which is consistent with the results of the HRTEM.
 | ||
| Fig. 2 TEM image (A) and corresponding size distribution (B), HRTEM image (C) and XRD pattern (D) of the CDs. | ||
The size of CDs could be easily tuned by controlling the reaction time. After refluxing for 2 h, the membranous structure of the carbonized β-cyclodextrin was torn apart and a stable black suspension formed. With increasing time, the size of the carbonized β-cyclodextrin pieces continued to reduce and CDs smaller than 10 nm took shape after 10 h. The reaction was refluxed until CDs with the desired size were obtained.
The UV-Vis absorption spectrum of the CDs is depicted in Fig. 3(A). The peak at 250–300 nm represents the typical absorption of an aromatic pi system, which is similar to that of polycyclic aromatic hydrocarbons.30Fig. 3(B) shows the photoluminescence spectrum of the CDs giving a green emission when excited at 340 nm. The inset photos were taken using a digital camera under natural light and UV irradiation respectively, which fits well to the FL spectrum. The quantum yield of the CDs with green emission is estimated to be about 6.4% by calibration against quinine sulfate.31 Compared with the quantum yields of some reported CDs synthesized by ultrasonic treatment (∼5%),9 hydrothermal method (∼2.4%)2 or laser irradiation (∼3%)5 as well as from nitric acid reflux of other carbon precursors (corn stalk soot: 0.8–1.9%, candle soot: 3%, natural gas soot: 0.43%),7,32,33 our method is effective and suitable for the synthesis of high quality CDs.
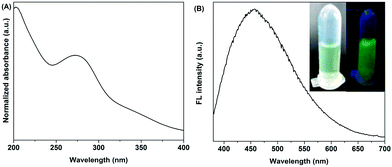 | ||
| Fig. 3 UV-Vis (A) and FL (B) absorption spectra of the CDs; the inset graphics are digital photos of CDs under natural light (left) and UV light (right) irradiation respectively. | ||
3.2. Mechanism
β-Cyclodextrin was chosen to prepare the carbon precursor because of its unique spatial structure. The structure of β-cyclodextrin is a conical cylinder. The primary hydroxyl groups are placed on one edge of the conical cylinder structure, whereas all the secondary ones are situated on the other edge.22 The cavity is composed of glycosidic oxygen bridges and hydrogen atoms, leading to hydrophobic characteristics.21 A possible reaction process is proposed as follows. Li and coworkers34 have proven that, when the reaction temperature is higher than 160 °C, intermolecular dehydration of the oligosaccharides or macromolecules takes place. Therefore, when β-cyclodextrin was calcined at 300 °C, the hydroxyl groups on the rings started to dehydrate and partially carbonize. Due to the special spatial structure, the carbonized blocks tended to form the membranous structure as illustrated in Scheme 1, which is the key factor for the formation of high-quality CDs in high yields.To better corroborate the reaction process, a control experiment was conducted using starch instead of β-cyclodextrin while keeping the other conditions the same. Starches are also polysaccharides composed of glucose residues, with the exception of the chain structure.21 The hydroxyl groups are evenly distributed on the chain, and can dehydrate and carbonize in any direction. Thus, the membranous structure was not found in the carbonized starch. The corresponding CDs prepared from carbonized starch were unevenly distributed with poor quality and low yield. Thus, it is believed that the special spatial structure of β-cyclodextrin facilitates the formation of high-quality CDs.
The presence of the defects on the membranous carbonized β-cyclodextrin makes it fragile and vulnerable to attack. CDs were obtained simply by refluxing in HNO3. With regard to the formation of the CDs, an unzipping mechanism has been proposed for the cutting process.35 During acid oxidization, the O atoms in the epoxy groups are removed, and then convert to more stable carbonyl groups, which fit with the IR results.
3.3. Reactivity tests
The as-synthesized CDs were found to possess peroxidase-like activity and oxidized a peroxidase substrate TMB in the presence of H2O2 to produce a blue color. Similar to natural enzymes (HRP for example), the catalytic activity of the CDs is affected by reaction conditions.36 Thus, the pH and temperature dependent-activity of the CDs and HRP were investigated. The maximum absorbance for the HRP sample in each curve (A–C) is set at 100% and is compared with the absorbance for the CDs.As shown in Fig. 4(A), the catalytic oxidation was faster in acidic solutions than in neutral or basic solutions which might be due to the good solubility of TMB in acidic solutions. Compared with HRP, the most suitable temperature for the CDs was about 45 °C. Furthermore, the catalytic activity of the CDs remained stable over a wider range. As previously reported, the enzyme-catalyzed reaction is often inhibited at high substrate concentrations. The same phenomenon was observed for the CDs (Fig. 4(C)). Yet, the CDs required a higher H2O2 concentration than HRP to reach maximal peroxidase activity. This indicates that at high H2O2 concentration, the catalytic activity of the CDs is more stable than that for HRP. The concentration of the CDs also affects the reaction activity and the optimum concentration of CDs is 10 μg mL−1. Therefore, the following reactions were conducted under the following optimal conditions: pH 4.5, 45 °C, and 10 μg mL−1 CDs.
 | ||
| Fig. 4 pH (A), temperature (B), H2O2 concentration (C) and CD concentration (D) response curve for H2O2 detection. | ||
As noted above, the color variation of the TMB oxidation catalyzed by CDs is related to the H2O2 concentration. The system discussed above could be used for the detection of H2O2. The concentration of TMB is 0.8 mM and the H2O2 concentration is varied and the error bars represent the standard error derived from three repeated measurements. Fig. 5(A) and (B) show typical H2O2 concentration–response curves. Under the optimal conditions, concentrations of H2O2 as low as 1 × 10−6 mol L−1 could be detected with a linear range from 2 × 10−6 to 5 × 10−4 mol L−1. The inserted graphics indicate the color evolution of the solution.
 | ||
| Fig. 5 Dose–response curve (A) and linear calibration plot (B) for H2O2 detection using CDs as an artificial enzyme mimetic. | ||
Aside from the detection of H2O2, we discovered that the above system could also be used for the colorimetric detection of some oxidative ions (such as Fe3+ and Ag+). Fe3+ and Ag+ solutions were used instead of H2O2 while keeping other conditions the same. Fig. 6(A) and (B) show the typical Fe3+ concentration–response curves where as low as 8 × 10−7 mol L−1 Fe3+ could be detected and the linear range was 8 × 10−6 to 1 × 10−4 mol L−1. For Ag+, the linear range was 5 × 10−6 to 1 × 10−4 mol L−1, with the detection limit of 5 × 10−7 mol L−1. Our result represents the first examples of ion detection utilizing the peroxidase-like activity of CDs. This special property could be used for rapid and sensitive detection of ions which might hold a great promise for sensors and environmental science and analysis.
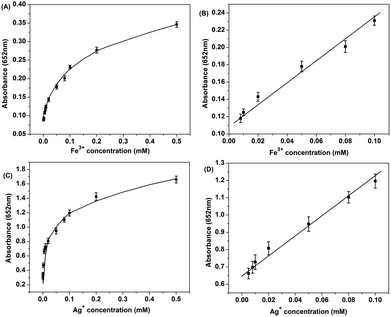 | ||
| Fig. 6 Dose–response curve (A) and linear calibration plot (B) for Fe3+ detection; dose–response curve (C) and linear calibration plot (D) for Ag+ detection. | ||
As shown by several groups, the mechanism of peroxidase-like activity follows the typical ping–pong mechanism.8,9,28,36 To investigate the mechanism of the peroxidase-like activity of the CDs for the detection of Ag+ and Fe3+, we measured the reaction kinetics over a range of TMB and Fe3+ (or Ag+) concentrations. Fig. 7 shows the typical double reciprocal plots of the initial velocity (V) versus one substrate concentration, which were obtained for a range of concentrations of the second substrate. It is evident that the slopes of the lines are parallel, which indicates a characteristic ping–pong mechanism, as was observed for HRP. This indicates that, similar to HRP, the CDs bind and react with one substrate, and release the first product before binding and reacting with the second substrate. It is well known that electron transfer occurs when graphene oxide or single-walled carbon nanotubes were used as a peroxidase mimetic.37,38 Raman scattering (Fig. 1(B)) indicates the existence of graphitic carbon in the CDs. Therefore we consider that a CD-promoted electron transfer may contribute to the catalytic ability of the CDs.
 | ||
| Fig. 7 Double reciprocal plots of CD activity with the concentration of one substrate (Fe3+/Ag+ or TMB) fixed and the other varied. | ||
To evaluate the selectivity of the CDs for H2O2, Ag+ and Fe3+ recognition, 1 mM K+, Na+, Zn2+, Fe2+, Cu2+, Pb2+, Ni2+, Cd2+, Al3+, and Cr3+ were added to the detection experiments for H2O2, Ag+ and Fe3+ respectively, and the absorbance changes with or without interfering ions were measured. The results are presented in Fig. 8, and show that the response of the CDs to H2O2, Ag+ and Fe3+ was almost unchanged before and after the addition of the interfering ions. These results demonstrate that our CDs are highly selective for the detection of H2O2, Ag+ and Fe3+.39
4. Conclusions
In summary, high-quality CDs were prepared from carbonized β-cyclodextrin and investigated as peroxidase mimetics. The CDs acted as peroxidase mimetics and provided a fast and sensitive colorimetric assay for H2O2. The detection limit toward H2O2 was 1 × 10−6 mol L−1 with a linear range from 2 × 10−6 to 5 × 10−4 mol L−1. More importantly, we expanded the application of peroxidase-like properties for CDs. A sensitive and fast analytical platform for oxidative ions, such as Fe3+ and Ag+ was developed. The detection limits for Fe3+ and Ag+ were 8 × 10−7 mol L−1 Fe3+ and 5 × 10−7 mol L−1 respectively, and the linear ranges were 5 × 10−6 to 1 × 10−4 mol L−1 and 8 × 10−6 to 1 × 10−4 mol L−1 respectively. Our work provided a simple synthesis route for the fast and economical preparation of high-quality CDs that can serve as peroxidase mimetics. Due to their easy preparation, robustness, and stability, the CDs can rival natural enzymes and find use in a variety of simple, robust and cost-effective biosensors and environmental science applications in the future.Acknowledgements
This work is supported financially by the National Natural Science Foundation of China (no. 51272107), and the Natural Science Foundation of Jiangsu Province, China (no. BK2011024).Notes and references
- X. Xu, R. Ray, Y. Gu, H. J. Ploehn, L. Gearheart and W. A. Scrivens, J. Am. Chem. Soc., 2004, 126, 12736–12737 CrossRef PubMed.
- Z. C. Yang, M. Wang, A. M. Yong, S. Y. Wong, X. Li and J. Wang, Chem. Commun., 2011, 47, 11615–11617 RSC.
- H. Ming, Z. Ma, Y. Liu, K. Pan, F. Wang and Z. Kang, Dalton Trans., 2012, 41, 9526–9531 RSC.
- L. Yuan, X. H. Lu, X. Xiao, T. Zhai, J. Zhou and Z. L. Wang, ACS Nano, 2012, 6, 656–661 CrossRef PubMed.
- S. L. Hu, K. Y. Niu, J. Sun, J. Yang, N. Q. Zhao and X. W. Du, J. Mater. Chem., 2009, 19, 484–488 RSC.
- J. C. Fan, H. H. Sung, C. R. Lin and M. H. Lai, J. Mater. Chem., 2012, 22, 9794–9797 RSC.
- Y. Li, L. Xu, T. Chen, X. Liu, Z. Xu and H. Zhang, Anal. Chim. Acta, 2012, 726, 102–108 CrossRef PubMed.
- S. Zhang, Q. He, R. Li, Q. Wang, Z. Hu, X. Liu and X. Chang, Mater. Lett., 2011, 65, 2371–2373 CrossRef PubMed.
- X. He, H. Li, Y. Liu, H. Huang, Z. Kang and S. T. Lee, Colloids Surf., B, 2011, 87, 326–332 CrossRef PubMed.
- H. Li, X. He, Y. Liu, H. Huang, S. Lian, S. T. Lee and Z. Kang, Carbon, 2011, 49, 605–609 CrossRef PubMed.
- H. Wu, C. Mi, H. Huang, B. Han, J. Li and S. Xu, J. Lumin., 2012, 132, 1603–1607 CrossRef PubMed.
- B. Zhang, C. Liu and Y. Liu, Eur. J. Inorg. Chem., 2010, 28, 4411–4414 CrossRef.
- H. Liu, T. Ye and C. Mao, Angew. Chem., 2007, 119, 6593–6595 CrossRef.
- H. Li, X. He, Y. Liu, H. Yu, Z. Kang and S. T. Lee, Mater. Res. Bull., 2011, 46, 147–151 CrossRef PubMed.
- D. Pan, J. Zhang, Z. Li, C. Wu, X. Yan and M. Wu, Chem. Commun., 2010, 46, 3681–3683 RSC.
- Y. Yang, J. Cui, M. Zheng, C. Hu, S. Tan, Y. Xiao, Q. Yang and Y. Liu, Chem. Commun., 2012, 48, 380–382 RSC.
- S. Sahu, B. Behera, T. K. Maiti and S. Mohapatra, Chem. Commun., 2012, 48, 8835–8837 RSC.
- A. Rahy, C. Zhou, J. Zheng, S. Y. Park, M. J. Kim, I. Jang, S. J. Cho and D. J. Yang, Carbon, 2012, 50, 1298–1302 CrossRef PubMed.
- B. E. Hamaoui, L. Zhi, J. Wu, J. Li, N. T. Lucas, Ž. Tomović, U. Kolb and K. Müllen, Adv. Funct. Mater., 2007, 17, 1179–1187 CrossRef.
- H. Zhu, X. Wang, Y. Li, Z. Wang, F. Yang and X. Yang, Chem. Commun., 2009, 34, 5118–5120 RSC.
- J. Szejtli, Chem. Rev., 1998, 98, 1743–1753 CrossRef CAS PubMed.
- E. M. Martin Del Valle, Process Biochem., 2004, 39, 1033–1046 CrossRef.
- Y. Y. Chu, Z. B. Wang, Z. Z. Jiang, D. M. Gu and G. P. Yin, Adv. Mater., 2011, 23, 3100–3104 CrossRef CAS PubMed.
- Y. Fang, S. Guo, D. Li, C. Zhu, W. Ren, S. Dong and E. Wang, ACS Nano, 2012, 6, 400–409 CrossRef CAS PubMed.
- A. B. Bourlinos, A. Stassinopoulos, D. Anglos, R. Zboril, V. Georgakilas and E. P. Giannelis, Chem. Mater., 2008, 20, 4539–4541 CrossRef CAS.
- S. N. Baker and G. A. Baker, Angew. Chem., Int. Ed., 2010, 49, 6726–6744 CrossRef CAS PubMed.
- L. Yuan, Y. Tao, J. Chen, J. Dai, J. Zhou and Z. L. Wang, Adv. Funct. Mater., 2011, 21, 2150–2154 CrossRef CAS.
- W. Shi, Q. Wang, Y. Long, Z. Cheng, S. Chen, H. Zheng and Y. Huang, Chem. Commun., 2011, 47, 6695–6697 RSC.
- Z. C. Yang, X. Li and J. Wang, Carbon, 2011, 49, 5207–5212 CrossRef CAS PubMed.
- S. Y. Xie, R. B. Huang and L. S. Zheng, J. Chromatogr. A, 1999, 864, 173–177 CrossRef CAS.
- D. F. Eaton, Pure Appl. Chem., 1988, 60, 1107–1114 CrossRef CAS.
- S. C. Ray, A. Saha, N. R. Jana and R. Sarkar, J. Phys. Chem. C, 2009, 113, 18546–18551 CAS.
- L. Tian, D. Ghosh, W. Chen, S. Pradhan, X. Chang and S. Chen, Chem. Mater., 2009, 21, 2803–2809 CrossRef CAS.
- X. Sun and Y. Li, Angew. Chem., Int. Ed., 2004, 43, 597–601 CrossRef PubMed.
- D. Pan, J. Zhang, Z. Li and M. Wu, Adv. Mater., 2010, 22, 734–738 CrossRef CAS PubMed.
- L. Gao, J. Zhuang, L. Nie, J. Zhang, S. Perrett and X. Li, Nat. Nanotechnology, 2007, 2, 577–583 CrossRef CAS PubMed.
- W. He, Y. Liu, J. Yin, X. Wu, X. Hu and C. Chen, Biomaterials, 2011, 32, 1139–1147 CrossRef CAS PubMed.
- Y. Song, K. Qu, C. Zhao, J. Ren and X. Qu, Adv. Mater., 2010, 22, 2206–2210 CrossRef CAS PubMed.
- A. Salinas-Castillo, M. Ariza-Avidad, C. Pritz, M. Camprubí-Robles, B. Fernández, M. J. Ruedas-Rama and A. Megia-Fernández, Chem. Commun., 2013, 49, 1103–1105 RSC.
| This journal is © The Royal Society of Chemistry 2014 |