Synthesis of chlorinated and anhydride-modified low density polyethylene by solid-phase chlorination and grafting—improving the adhesion of a film-forming polymer
Abstract
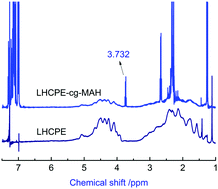
This paper is mainly focused on the modification of low density polyethylene (LDPE) by chlorinating and grafting it simultaneously in the gas–solid phase. There are two key obstacles to the success of this process. The first is the feasibility of the chlorination and grafting occurring simultaneously on the LDPE chains, and the second is how to gain control of the high chlorinated polyethylene (HCPE, Clwt% > 60%) preparation, when using LDPE as the raw material, which is a heterogeneous reaction. The feasibility of the process is discussed by FTIR and 1H-NMR, in which LDPE is chlorinated to a high chlorine content (Clwt% = 64%) while maleic anhydride (MAH) is grafted onto the backbone chain (LDPE). This is a novel modification method patented by us, called in situ chlorinating graft copolymerization (ISCGC). The products’ molecular weight and distribution, thermal properties, and the factors affecting the modification process are analyzed by gel permeation chromatography (GPC), differential scanning calorimetry (DSC), stereoscopic microscopy and the curve of chlorine content versus time. A graft degree (GD) of about 2% is achieved for the graft copolymer, which has a broad practical significance. The adhesion and impact strength tests of the product as a film-forming polymer in coatings indicate that it could effectively increase the adhesion when MAH is grafted onto the LDPE by ISCGC.

 Please wait while we load your content...
Please wait while we load your content...