Density Functional Theory study of the structural and electronic properties of H3PO4/ZSM-5
Yanping Huang,
Xiuqin Dong,
Mengmeng Li,
Minhua Zhang* and
Yingzhe Yu*
Key Laboratory for Green Chemical Technology of Ministry of Education, R&D Center for Petrochemical Technology, Tianjin University, Tianjin 300072, P.R. China. E-mail: mhzhang@tju.edu.cn; yzhyu@tju.edu.cn; Fax: +86-22-27406119; Fax: +86-22-27406119; Tel: +86-22-27406119 Tel: +86-22-27405972
First published on 30th January 2014
Abstract
In this work, a Density Functional Theory (DFT) study has been carried out to investigate the structural and electronic properties of H3PO4/ZSM-5 (extra-framework and framework modification). Cluster models at different T sites (T6, T9, and T12) are suggested. The local structure of the extra-framework modified cluster for H3PO4/ZSM-5 shows that there is a hydrogen bond interaction between the hydrogen atoms in H3PO4 and the oxygen atoms in the zeolite framework, involving O4 and Ha. Additionally, the Ozeo always tends to keep in a straight line distribution. Mulliken charge analysis of the cluster models concerned shows that the charge indeed transfers from the oxygen atoms toward P, Al and H atoms. The charge transfer from ZSM-5 to H3PO4 could enhance the interaction between the H3PO4 and the ZSM-5. Based on the DFT study above, the mutual relationship between the acidic sites on ZSM-5 and phosphoric acid might be more clearly confirmed.
1. Introduction
It has long been known that bio-ethanol reacts with HZSM-5 zeolite (MFI) to give a mixture of hydrocarbons which are rich in ethylene, called bio-ethanol to ethylene (B.E.T.E.).1–5 Zeolites play an important role in catalytic reactions in the chemical industry. Studies involving zeolites are attracting the attention of global researchers.6–8 A number of works have reported that the introduction of phosphorus acid into HZSM-5 by wet impregnation could provide the catalyst with a much lower selectivity for aromatic byproducts, and a lower reaction temperature.9–15Many experiments have confirmed the existence of interactions between H3PO4 and the solid acid of HZSM-5. Abubakar et al.9 analyzed the structure and mechanism of phosphate-modified HZSM-5 for methanol conversion. It was found that (1) XRD revealed no bulk phases other than the zeolite; (2) 27Al, 29Si, and 1H MAS (magic-angle spinning)-NMR all showed extensive dealumination of the zeolite framework from an initial SiO2/Al2O3 ratio of 80 to a final value that was typically near 240; and (3) 31P MAS-NMR showed that, with the presence of water and methanol, phosphorus was present in the modified zeolite as phosphoric acid. However, through dehydration or calcination, it was present as P4O10. Ramesh et al.11,15 studied a P-modified HZSM-5 catalyst in selective ethanol dehydration. In their results, 27Al MAS NMR spectra suggested that the addition of P facilitates the cleavage of the Si–O–Al bond, which leads to partial dealumination. The NH3-TPD results indicated that the total acidity and the amount of strong acid sites decreases with P loading.
At present, five framework modification models have been proposed through experiments (as shown in Fig. 1). As early as 1986, Lercher et al.18 proposed a model to describe the interaction between the bridging hydroxyls of ZSM-5 and orthophosphoric acid. Corma et al.19 summarized the models present in the literature for describing the interaction between HZSM-5 and phosphorous species, for elucidation of the status of the phosphorus species and the hydroxyl groups. Xue et al.20 proposed the mechanism of the interaction between phosphorus and HZSM-5. Lü et al.21 used DFT to investigate the five models, and found that among the five proposed models, model B (where the terminal oxygen of the phosphorus species interacts with aluminum) is the most plausible model for describing the phosphorus species grafting on a zeolite framework. However, Lü et al.21 didn't fix any atoms in the five models, and the optimized structures could not maintain the ZSM-5 channels. So, the terminal H atoms of the models in this study were fixed in order to maintain the ZSM-5 crystal.
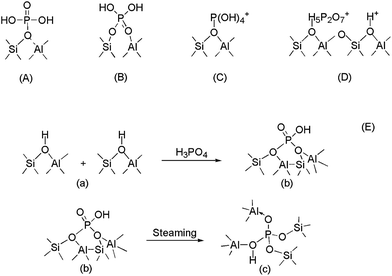 | ||
| Fig. 1 Models proposed for the interaction of phosphorus with the Brönsted acid sites of HZSM-5.20 (A) proposed by Kaeding et al.16 and Védrine et al.;17 (B) proposed by Lercher et al.,18 (C) proposed by Corma et al.;19 (E) proposed by Xue et al.20 | ||
In this work, a DFT study was carried out in order to understand the structural and electronic properties of H3PO4/ZSM-5. The main aims of this work are: (1) to investigate the local structural configuration of the extra-framework and framework modified HZSM-5; (2) to elucidate the electronic properties of the extra-framework and framework modified HZSM-5; (3) to understand the inherent relationship between H3PO4 and the acidic sites on the HZSM-5.
2. Computational models and methods
2.1 Cluster selection
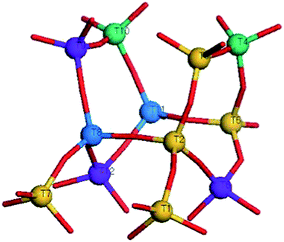
ZSM-5 has an MFI framework topology, characterized by a three-dimensional pore system with straight and sinusoidal channels. The pore vacancies are defined by 10 member oxygen-rings that are wide enough (about 5.5 Å) to allow molecules as large as benzene to pass through. As shown in Fig. 2, among the twelve crystallographic distinct T sites, the T4 and T10 sites (marked in green) only appear in the 10 member O-ring which forms the straight channel. Similarly, the T8 and T11 sites (marked in blue) only appear in the 10 member O-ring which forms the sinusoidal channel. Thus, there are eight T sites which are common to both straight and sinusoidal channels.22,23 Many studies have reported that the T6, T9 and T12 sites (marked in purple) are the most stable positions for the Al atom,24–26 but some other studies have suggested that the T12 site is the most stable position for the Al atom.27–30In this work, 8T (H3SiO)3Si–O(H)–T(OSiH3)3(T![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Si, Al) cluster models are applied to investigate the modifications at the T6, T9, and T12 sites, respectively. The terminal Si atoms are saturated with H atoms, and all terminal Si–H bond lengths r(Si–H) are fixed at 1.470 Å along the direction of the Si–O bond, as determined from crystallographic data.31 To allow for site relaxation upon Al (P) substitution, only the terminal H atoms are fixed at crystallographic locations. The initial configuration of ZSM-5 is taken from the siliceous ZSM-5 crystal.
Si, Al) cluster models are applied to investigate the modifications at the T6, T9, and T12 sites, respectively. The terminal Si atoms are saturated with H atoms, and all terminal Si–H bond lengths r(Si–H) are fixed at 1.470 Å along the direction of the Si–O bond, as determined from crystallographic data.31 To allow for site relaxation upon Al (P) substitution, only the terminal H atoms are fixed at crystallographic locations. The initial configuration of ZSM-5 is taken from the siliceous ZSM-5 crystal.
2.2 Computational methods
All calculations are performed using the DMol3 program from MS (Materials Studio).32,33 The double numerical plus polarization (DNP) basis set is used in the calculation to describe the valence orbital of all of the atoms concerned. The numerical basis sets in DMol3 minimize or even eliminate basis set superposition error (BSSE), in contrast to Gaussian basis sets, where BSSE can be a serious problem.34,35 The DFT study with the DMol3 module is performed with the generalized gradient corrected approximation (GGA) using the BLYP functional.36,37 The advantage of the BLYP method employed in this study is that it has a correction term for dispersion forces.38 In this study, no symmetry constraints are used for any cluster models. The atom charges are calculated using the approach proposed by Mulliken.39The relative stability of the H3PO4 in each extra-framework site is evaluated by calculating the binding energy (BE):
| Ebind = −{E(H3PO4/HZSM-5) − E(H3PO4) − E(HZSM-5)} |
The deprotonation energy (DE) is calculated to estimate the acidity of the bridging hydroxyl groups, which is expressed as the total energy difference between the initial (neutral) and the deprotonated (anionic) forms of the HZSM-5 or H3PO4/HZSM-5 clusters. The DE value cannot be directly measured experimentally. The difference between the DE values of two different OH groups can be determined from the difference in the binding energies of a probe basic molecule.40
3. Results and discussion
3.1 Structural configurations of H3PO4/HZSM-5
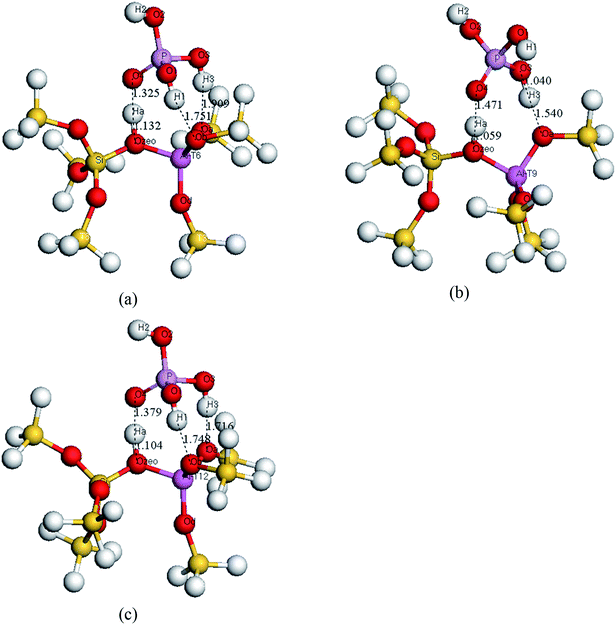 | ||
| Fig. 3 Optimized structural configurations of extra-framework modified H3PO4/HZSM-5 cluster models. (a) T6, (b) T9 and (c) T12. | ||
| Sites | Bond name and length, Å | Bond angle, deg. | ||||
|---|---|---|---|---|---|---|
| P–O | O–H | Ozeo–Ha | Al–O | Al–O–Si | ||
| a O1–O4 are denoted as the individual oxygen atoms in H3PO4. Oa, Ob, Ozeo are denoted as the individual framework oxygen atoms in HZSM-5. | ||||||
| T6 | P–O1 1.597 | O1–H1 1.013 O2–H2 0.975 | 1.132 | Al–Oa 1.743 | Init.134.569 | |
| P–O2 1.598 | O3–H3 0.999 O4–Ha 1.325 | Al–Ob 1.747 | Opt.133.488 | |||
| P–O3 1.590 | Oa–H3 1.909 Ob–H1 1.751 | Al–Ozeo 1.807 | ||||
| P–O4 1.521 | Al–Od 1.693 | |||||
| T9 | P–O1 1.625 | O1–H1 0.977 O2–H2 0.975 | 1.059 | Al–Oa 1.759 | Ini.133.404 | |
| P–O2 1.607 | O3–H3 1.040 O4–Ha 1.471 | Al–Ob 1.707 | Opt.131.276 | |||
| P–O3 1.569 | Oa–H3 1.540 Ob–H1 5.712 | Al–Ozeo 1.845 | ||||
| P–O4 1.514 | Al–Od 1.709 | |||||
| T12 | P–O1 1.599 | O1–H1 1.009 O2–H2 0.975 | 1.104 | Al–Oa 1.733 | Init.128.060 | |
| P–O2 1.602 | O3–H3 1.011 O4–Ha 1.379 | Al–Ob 1.738 | Opt.126.010 | |||
| P–O3 1.587 | Oa–H3 1.716 Ob–H1 1.748 | Al–Ozeo 1.814 | ||||
| P–O4 1.521 | Al–Od 1.691 | |||||
It can be seen that the proton Ha in the bridging hydroxyl group migrates toward the O4 atom in the H3PO4 for all extra-framework modified cluster models. Before modification, the Ozeo–Ha bond length in the HZSM-5 is about 0.977 Å. After the introduction of H3PO4 into ZSM-5, the Ozeo–Ha bond length is extended to 1.059 Å, 1.104 Å and 1.132 Å for different models, while the distance between O4 atom in H3PO4 and Ha atom is 1.325 Å, 1.379 Å and 1.471 Å for different models. This indicates the existence of a hydrogen bond interaction between the O4 and Ha atoms. This interaction plays a vital role in the thorough dispersion of the H3PO4, resulting in a better catalytic performance. Additionally, O4, Ha and Ozeo are always situated on the same straight line.
The distances between the hydrogen atoms in the H3PO4 and the framework oxygen atoms are shown in Table 1. It can be seen that the Oa–H3 and Ob–H1 bond distances range from 1.716 to 1.909 Å. This suggests the existence of hydrogen bond interactions between the hydrogen atoms in H3PO4 and the framework oxygen atoms in the zeolite. For the T9 site, only the Oa and H3 atoms show a hydrogen bond interaction. Such results cannot be experimentally obtained since the EXAFS technique can only determine the average bond distance.41 This information might be very important for exploring the reaction mechanism.
The interaction between the H3PO4 and the HZSM-5 will cause some changes in the Al–O bond length and the Al–O–Si bond angle. The Al–O bond lengths are listed in Table 1. For all cluster models, the bond lengths between Al and the Oa, Ob and Ozeo atoms are longer than that of Al–Od. This can be attributed to the strong hydrogen bond interactions between the hydrogen atoms in H3PO4 and the oxygen atoms in the zeolite framework. The Al–O–Si bond angles are also shown in Table 1. Redondo et al.24 reported that larger T–O–T bond angles could represent a stronger acidity (lower proton affinity). The optimized Al–O–Si bond angles for all cluster models are smaller than those for the initial HZSM-5, which might indicate that the acidity of the zeolite decreases after the H3PO4 extra-framework is modified. This is consistent with the NH3-TPD results.11,15 The sequence of acid strength (from weak to strong) at different T sites is: T12 < T9 < T6.
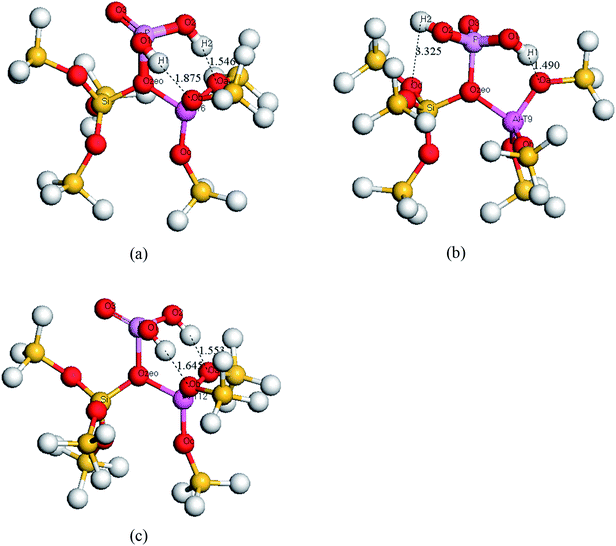 | ||
| Fig. 4 Optimized structural configurations of the framework modified H3PO4/HZSM-5 cluster models. (a) T6, (b) T9, (c) T12. | ||
| Sites | Bond name and length, Å | Bond angle, deg. | |||
|---|---|---|---|---|---|
| P–O | O–H | Al–O | P–O–Si | ||
| a O1–O4 are denoted as the individual oxygen atoms in H3PO4. Oa, Ob, Ozeo aredenoted as the individual framework oxygen atoms in HZSM-5. | |||||
| T6 | P–O1 1.594 P–O2 1.575 | Ob–H1 1.875 | Al–Oa 1.550 | Init. 134.569 | |
| P–O3 1.471 P–Ozeo 1.851 | Oa–H2 1.546 | Al–Ob 1.598 | Opt 122.097 | ||
| Al–Oc 1.602 | |||||
| Al–Ozeo 1.588 | |||||
| T9 | P–O1 1.608 P–O2 1.564 | Oa–H1 1.490 | Al–Oa 1.606 | Init. 134.404 | |
| P–O3 1.475 P–Ozeo 1.822 | Od–H2 3.325 | Al–Ob 1.608 | Opt. 119.292 | ||
| Al–Oc 1.612 | |||||
| Al–Ozeo 1.709 | |||||
| T12 | P–O1 1.585 P–O2 1.575 | Ob–H1 1.645 | Al–Oa 1.727 | Init. 128.060 | |
| P–O3 1.470 P–Ozeo 1.846 | Oa–H2 1.553 | Al–Ob 1.729 | Opt.116.809 | ||
| Al–Oc 1.683 | |||||
| Al–Ozeo 1.884 | |||||
As shown in Fig. 4, the optimized structures turn out to be the same as the structure of model A in Fig. 1. The distance between the hydrogen atoms in the H3PO4 and the framework oxygen atoms are shown in Table 1. It can be seen that the Ob–H1 and Oa–H2 bond distances are from 1.490, 1.645 and 1.875 Å, respectively. This suggests the existence of a hydrogen bond interaction between the hydrogen atoms in the H3PO4 and the framework oxygen atoms in the zeolite. For the T9 site, only the Oa and H1 atoms show a hydrogen bond interaction.
3.2 Electronic properties of H3PO4/HZSM-5
The Mulliken population analyses of the P, Al, O atoms (the zeolite framework oxygen atoms and the oxygen atoms in H3PO4), and H atoms (the proton hydrogen atom and the hydrogen atoms in H3PO4) are listed in Table 3.| Sites | Atoms and Mulliken charge, |e| | ||||
|---|---|---|---|---|---|
| P | O | H | Al | ||
| T6 | 1.519 | O1 - 0.604 O2 - 0.527 O3 - 0.570 | H1 0.354H2 0.288 | 1.350 | |
| O4 - 0.725 Oa - 0.919 Ob - 0.914 | H3 0.356 Ha 0.490 | ||||
| T9 | 1.494 | O1 - 0.553 O2 - 0.528 O3 - 0.604 | H1 0.287H2 0.286 | 1.374 | |
| O4 - 0.755 Oa - 0.942 Ob - 0.846 | H3 0.403 Ha 0.465 | ||||
| T12 | 1.508 | O1 - 0.598 O2 - 0.529 O3 - 0.590 | H1 0.357H2 0.285 | 1.362 | |
| O4 - 0.744 Oa - 0.926 Ob - 0.919 | H3 0.369 Ha 0.470 | ||||
The Mulliken net charges of the framework oxygen atoms and the oxygen atoms in H3PO4 are also listed in Table 3. It can be seen that there is a charge transfer from the O atoms to the P, Al and H atoms. The charge transfer from the ZSM-5 to the P and H atoms in the H3PO4 could enhance the interaction between the H3PO4 and the ZSM-5.
The Mulliken population analyses of the framework modified cluster models are listed in Table 4. A similar conclusion could be obtained from the Mulliken net charges analysis, i.e. that a charge transfer from the O atoms to the P, Al and H atoms occurs.
| Sites | Atoms and Mulliken charge, |e| | ||||
|---|---|---|---|---|---|
| P | O | H | Al | ||
| T6 | 1.547 | O1 - 0.562 O2 - 0.610 O3 - 0.600 | H1 0.331H2 0.387 | 1.380 | |
| Ozeo - 0.984 Oa - 0.928 Ob - 0.907 | |||||
| T9 | 1.495 | O1 - 0.548 O2 - 0.586 O3 - 0.586 | H1 0.299H2 0.385 | 1.411 | |
| Ozeo - 0.965 Oa - 0.893 Ob - 0.849 | |||||
| T12 | 1.523 | O1 - 0.580 O2 - 0.597 O3 - 0.603 | H1 0.359H2 0.375 | 1.388 | |
| Ozeo - 0.968 Oa - 0.924 Ob - 0.921 | |||||
3.3 Deprotonation energy (DE) and binding energy (BE) of H3PO4/HZSM-5
The deprotonation energies and binding energies for each T site are shown in Table 5, where ΔED = EAl–O–Si − EAl–OH–Si.| Sites | Deprotonation energies, kcal mol−1 | BEext, kcal mol−1 | |||
|---|---|---|---|---|---|
| ΔED-un | ΔED-ext | ΔED-fra | |||
| a ΔED-un, ΔED-ext and ΔED-fra represent the deprotonation energies of unmodified, extra-framework modified, and framework modified zeolite, respectively. BEext represents the binding energy of extra-framework modified zeolites. | |||||
| T6 | 293.5662 | 295.6677 | 302.4867 | 21.0946 | |
| T9 | 294.7945 | 295.7426 | 302.6203 | 21.6091 | |
| T12 | 298.1843 | 298.4150 | 304.1262 | 25.6921 | |
A higher BE value indicates a higher stability of the configuration. By comparing the BE value at different T sites, it can be found that the most stable extra-framework modified cluster model is that at the T12 site, for which the BE value is about 4 kcal mol−1, higher than for the other models.
It is well known that the acidity of the catalyst is important for ethanol dehydration.42 The catalytic performance of the H3PO4 is highly dependent on the amount of acid in the ZSM-5. The deprotonation energies (DE) could be used as an effective method to evaluate the acidity of the zeolite.43,44,45 The lower the DE value of an OH group is, the stronger its Brönsted acidity is. As shown in Table 5, the ΔED-ext and ΔED-fra values are higher than the ΔED-un value, which suggests that these modifications could decrease the Brönsted acid strength of ZSM-5. The obtained results for DE are rather consistent with the T–O–T bond angles discussed above. For the extra-framework cluster models, an H3PO4H+ group is formed when the proton migrates toward the O4 atom in the H3PO4. Therefore, these cluster models can be considered as a proton H substituted by H3PO4H+.
3.4 Probe molecules
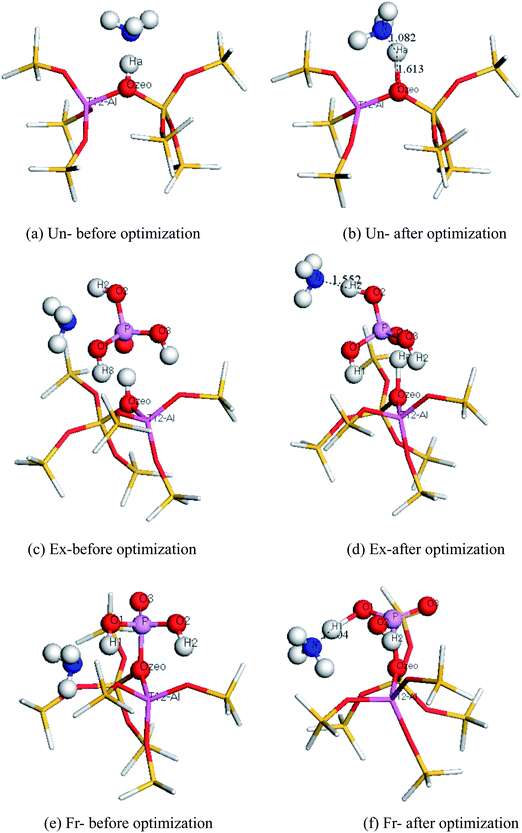
The acidity information for a zeolite can be obtained through the interaction of a basic probe molecule with the acid sites of the zeolite. In this study, DFT was used to calculate the interaction of the probe molecules and ZSM-5, to obtain the acidity information for ZSM-5. The stable adsorption configuration of the probe molecule NH3 adsorbed on the T12 site of ZSM-5 is shown in Fig. 5. In Fig. 5, blue represents N atoms, yellow represents Si atoms, red represents O atoms, deep purple represents Al atoms, light purple represents P atoms and white represents H atoms.As can be seen from Table 6, the ΔEInt of the unmodified ZSM-5 is the largest. The ΔEInt of the extra-framework modified ZSM-5 is smaller, and the ΔEInt of the framework modified ZSM-5 is the smallest. Thus, the Bronsted acidity of ZSM-5 in descending order is Un > Ex > Fr (that is, unmodified > extra-framework modified > framework modified), which is in agreement with our previously discussed results for deprotonation energies, and the experimental results of Tynjälä et al.46
| Model | ET (Ha) | ENH3 (Ha) | ET-NH3 (Ha) | ΔEInt (kcal mol−1) |
|---|---|---|---|---|
| Un | −2807.7385 | −56.5578 | −2864.3314 | 21.98 |
| Ex | −3452.0915 | −56.5578 | −3508.6835 | 21.48 |
| Fr | −3375.5873 | −56.5578 | −3432.1783 | 20.81 |
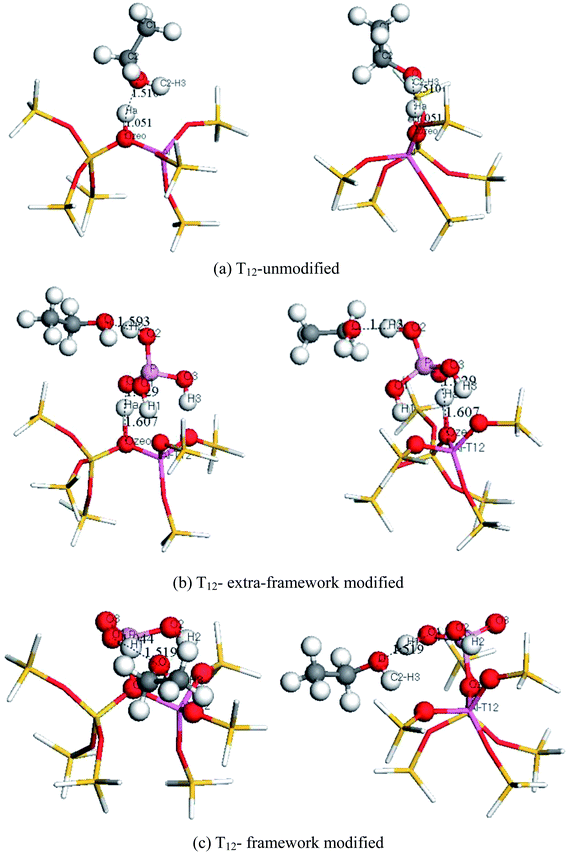
3.5 Ethanol adsorption
The stable configuration models of ethanol adsorption on the zeolite before and after P modification are shown in Fig. 6. The structural geometry parameters and the adsorption energy of the zeolite model when ethanol molecules are adsorbed on the zeolite before and after P modification are shown in Table 7. The adsorption energy of ethanol can be found with the following formula:| Ea = ET-C2H5OH − ET – EC2H5OH |
| 1Ha = 627.51 kcal mol−1. |
| Model | Ozeo–Ha (Å) | O–Ha (Å) | ET (Ha) | EC2H5OH (Ha) | ET-C2H5OH (Ha) | Ea (kcal mol−1) |
|---|---|---|---|---|---|---|
| Un | 1.051 | 1.510 | −2807.7426 | −155.0447 | −2962.8152 | −17.50 |
| Ex | 1.027 | 1.593 | −3452.0915 | −155.0447 | −3607.1607 | −15.44 |
| Fr | 1.044 | 1.519 | −3375.5873 | −155.0447 | −3530.6532 | −12.33 |
From Table 7, the adsorption energy of ethanol molecules before and after modification are −17.50 kcal mol−1, −15.44 kcal mol−1 and −12.33 kcal mol−1, respectively.
It can be seen that the ethanol always tends to form a hydrogen bond with the H2 atom in the H3PO4, but not with the original Ha atom in the ZSM-5. Therefore it could be speculated that after the introduction of phosphorus acid into the HZSM-5, the Brønsted acid proton could be considered as the H2 atom in H3PO4. This could explain the high stability of H3PO4/HZSM-5.
4. Conclusions
In this work, a DFT study confirms the hydrogen bond interactions between hydrogen atoms in H3PO4 and the framework oxygen atoms in ZSM-5, with the O4, Ha and Ozeo atoms always distributed in the same straight line. This configuration is the most stable structure for extra-framework modified HZSM-5. For framework modified models, the optimized structure is consistent with model A. The DFT study also confirms that a close relationship exists between the acid sites of the ZSM-5 and the H3PO4. It is suggested that the acid strength of the ZSM-5 is weakened after H3PO4 modification, which is consistent with the NH3-TPD results. The hydrogen bond interaction between the H3PO4 and the framework oxygen atoms in the ZSM-5 could result in the formation of an H3PO4H+ group, and thus the acidic sites on the ZSM-5 could keep the highly dispersed state of the H3PO4. It is speculated that after the introduction of the H3PO4 into the HZSM-5, the Brønsted acid proton could be considered as the H2 atom in the H3PO4.References
- R. Le Van Mao, T. M. Nguyen and G. P. McLaughlin, Appl. Catal., 1989, 48, 265–277 CrossRef CAS.
- W. R. Moser, R. W. Thompson, C.-C. Chiang and H. Tong, J. Catal., 1989, 117, 19–32 CrossRef CAS.
- T. M. Nguyen and R. Le Van Mao, Appl. Catal., 1990, 58, 119–129 CrossRef CAS.
- I. Takahara, M. Saito, M. Inaba and K. Murata, Catal. Lett., 2005, 105, 249–252 CrossRef CAS.
- I. Takahara, M. Saito, H. Matsuhashi, M. Inaba and K. Murata, Catal. Lett., 2007, 113, 82–85 CrossRef CAS.
- N. Wang, M. Zhang and Y. Yu, RSC Adv., 2014, 4, 4324–4329 RSC.
- X. Q. Zhang, R. A. van Santen and A. P. J. Jansen, Phys. Chem. Chem. Phys., 2012, 14, 11969–11973 RSC.
- X. Q. Zhang, T. T. trinh, R. A. van Santen and A. P. J. Jansen, J. Am. Chem. Soc., 2011, 133, 6613–6625 CrossRef CAS PubMed.
- S. M. Abubakar, D. M. Marcus, J. C. Lee, J. O. Ehresmann, C. Y. Chen, P. W. Kletnieks, D. R. Guenther, M. J. Hayman, M. Pavlova, J. B. Nicholas and J. F. Haw, Langmuir, 2006, 22, 4846–4852 CrossRef CAS PubMed.
- D. S. Zhang, R. Wang and X. X. Yang, Catal. Lett., 2008, 124, 384–391 CrossRef CAS.
- K. Ramesh, L. M. Hui, Y. F. Han and A. Borgna, Catal. Commun., 2009, 10, 567–571 CrossRef CAS.
- N. N. Zhan, Y. Hu, H. Li, D. H. Yu, Y. W. Han and H. Huang, Catal. Commun., 2010, 11, 633–637 CrossRef CAS.
- Z. X. Song, A. Takahashi, I. Nakamura and T. Fujitani, Appl. Catal., A, 2010, 384, 201–205 CrossRef CAS.
- N. H. Xue, R. Olindo and J. A. Lercher, J. Phys. Chem. C, 2010, 114, 15763–15770 CAS.
- K. Ramesh, C. Jie, Y. F. Han and A. Borgna, Ind. Eng. Chem. Res., 2010, 49, 4080–4090 CrossRef CAS.
- W. W. Kaeding and S. A. Butter, J. Catal., 1980, 61, 155–164 CrossRef CAS.
- J. C. Védrine, A. Auroux, P. Dejaifve, V. Ducarme, H. Hoser and S. Zhou, J. Catal., 1982, 73, 147–160 CrossRef.
- J. A. Lercher and G. Rumplmayr, Appl. Catal., 1986, 25, 215–222 CrossRef CAS.
- T. Blasco, A. Corma and J. Martínez-Triguero, J. Catal., 2006, 237, 267–277 CrossRef CAS.
- N. Xue, X. Chen, L. Nie, X. Guo, W. Ding, Y. Chen, M. Gu and Z. Xie, J. Catal., 2007, 248, 20–28 CrossRef CAS.
- R. Lü, Z. Cao and S. Wang, J. Mol. Struct.: THEOCHEM, 2008, 865, 1–7 CrossRef.
- A. Chatterjee and R. Vetrivel, J. Chem. Soc., Faraday Trans., 1995, 91, 4313–4319 RSC.
- A. Chatterjee and R. Vetrivel, J. Mol. Catal. A: Chem., 1996, 106, 75–81 CrossRef CAS.
- A. Redondo and P. J. Hay, J. Phys. Chem., 1993, 97, 11754–11761 CrossRef CAS.
- A. Chatterjee and A. K. Chandra, J. Mol. Catal. A: Chem., 1997, 119, 51–56 CrossRef CAS.
- M. S. Stave and J. B. Nicholas, J. Phys. Chem., 1995, 99, 15046–15061 CrossRef CAS.
- J. G. Fripiat, F. Berger-André, J.-M. André and E. G. Derouanc, Zeolites, 1983, 3, 306–310 CrossRef CAS.
- E. G. Derouane and J. G. Fripiat, Zeolites, 1985, 5, 165–172 CrossRef CAS.
- S. R. Lonsinger, A. K. Chakraborty, D. N. Theodorou and A. T. Bell, Catal. Lett., 1991, 11, 209–217 CrossRef CAS.
- A. Chatterjee and R. Vetrivel, Microporous Mater., 1994, 3, 211–218 CrossRef CAS.
- H. van Koningsveld, H. van Bekkum and J. C. Jansen, Acta Crystallogr., Sect. B: Struct. Sci., 1987, 43, 127–132 CrossRef.
- B. Delley, J. Chem. Phys., 1990, 92, 508–517 CrossRef CAS.
- B. Delley, J. Chem. Phys., 2000, 113, 7756–7764 CrossRef CAS.
- M. Elanany, M. Koyama, M. Kubo, P. Selvam and A. Miyamoto, Microporous Mesoporous Mater., 2004, 71, 51–56 CrossRef CAS.
- B. Kalita and R. C. Deka, Eur. Phys. J. D, 2009, 53, 51–58 CrossRef CAS.
- A. D. Becke, Phys. Rev. A, 1988, 38, 3098–3100 CrossRef CAS.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS.
- A. F. Jalbout, A. J. Hameed, F. I. Jimenez and M. Ibrahim, J. Organomet. Chem., 2008, 693, 216–220 CrossRef CAS.
- R. S. Mulliken, J. Chem. Phys., 1955, 23, 1833–1840 CrossRef CAS.
- G. N. Vayssilov and N. Rösch, J. Phys. Chem. B, 2001, 105, 4277–4284 CrossRef CAS.
- B. Wichtelová, Z. Sobalík and J. Dedecek, Appl. Catal., B, 2003, 41, 97–114 CrossRef.
- K. M. Abd El-Salaam and E. A. Hassan, Surf. Technol., 1982, 16, 121–128 CrossRef CAS.
- N. Gonzales, A. Chakraborty and A. Bell, Catal. Lett., 1998, 50, 135–139 CrossRef CAS.
- M. Sierka, U. Eichler, J. Datka and J. Sauer, J. Phys. Chem. B, 1998, 102, 6397–6404 CrossRef CAS.
- N. O. Gonzales, A. T. Bell and A. K. Chakraborty, J. Phys. Chem. B, 1997, 101, 10058–10064 CrossRef CAS.
- P. Tynjala and T. T. Pakkanen, Microporous Mesoporous Mater., 1998, 20, 363–369 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |