Michael addition of pyrimidine derivatives with acrylates catalyzed by lipase TL IM from Thermomyces lanuginosus in a continuous-flow microreactor†
Abstract
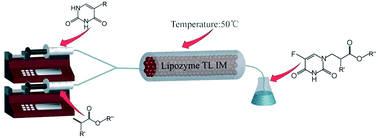
Lipase-catalyzed Michael addition of pyrimidine derivatives to acrylates in a continuous-flow microreactor is described. The influence of the structure of the Michael acceptor and the corresponding donor on the enzymatic addition was also investigated. The important features of this method include mild reaction conditions, short reaction times (30 min) and high yields.

 Please wait while we load your content...
Please wait while we load your content...