Aqueous zinc ammine complex for solution-processed ZnO semiconductors in thin film transistors†
Abstract
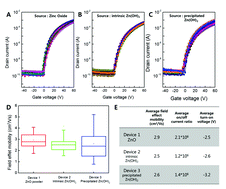
We fabricated zinc oxide (ZnO) TFTs using a zinc ammine complex with various zinc oxide sources such as ZnO, intrinsic Zn(OH)2, and precipitated Zn(OH)2. From the analyses of the reaction mechanism, surface morphology, crystal structure, and oxygen vacancy in the ZnO films, we confirmed the same intermediate in ZnO semiconductor films irrespective of the type of zinc oxide source in the zinc ammine complex precursor. The results showed the analogous value of the average field effect mobility, on/off current ratio, and turn-on voltage in all solution-processed ZnO TFTs. In conclusion, we confirmed that directly dissolving pristine ZnO into ammonia water is the most efficient method for preparing the ZnO semiconductor precursor, the zinc ammine complex, for low-temperature, solution-processed, and high performance ZnO TFTs.

 Please wait while we load your content...
Please wait while we load your content...