Microwave-promoted regio- and stereoselective vinylation of heterocyclic thiols†
Nimmakuri Rajesha,
Rupam Sarmab and
Dipak Prajapati*a
aMedicinal Chemistry Division, CSIR-North-East Institute of Science and Technology, Jorhat, Assam 785006, India. E-mail: dr_dprajapati2003@yahoo.co.uk; Fax: +91 376 2370011
bDepartment of Chemistry, Nalbari College, Nalbari, Assam 781335, India
First published on 6th January 2014
Abstract
The first stereoselective vinylation of heterocyclic thiols has been reported. Heterocyclic thiols are shown to react efficiently with activated terminal alkynes in an anti-Markovnikov fashion in absence of any catalyst or additive under microwave irradiation to give Z-selective vinyl sulfides.
Introduction
Vinyl sulfides are an important class of molecules as they serve as versatile synthons in natural product chemistry1 and are found in many biologically active molecules.2 They also find applications as Michael acceptors3 and enolate ion equivalents.4 There are two distinct and widely practiced routes for construction of vinyl sulfides:5 (i) by coupling of vinyl halides with thiols and (ii) by hydrothiolation of alkynes. Although there are numerous reports of synthesis of vinyl sulfides by either of the two pathways, control on the stereoselectivity of the reaction involves considerable synthetic challenge. For example, vinyl halides can be coupled with thiols in presence of copper(I) catalysts and various additives and ligands to obtain vinyl sulfides.6 The primary drawback of this method is the requirement of stereoselective starting compounds to carry out the transformation. In a way similar to vinyl halides, β-nitrostyrene can also be coupled with thiols to afford vinyl sulfides as mixture of E/Z compounds.7 However, inert atmosphere and an initiator are required for improved yield of the products. On the other hand, hydrothiolation of terminal alkynes is the more common and pursued method for preparing vinyl sulfides and as such there are numerous reports of this method under various conditions.8 Hammond and coworkers8b reported a green method for synthesizing vicinal dithioethers from terminal alkynes in aqueous media in moderate to good yields. Yang and Rioux8a developed a method for hydrothiolation of terminal alkynes in presence of a rhodium catalyst but obtained the products as E/Z mixture. Alternatively, hydrothiolation of alkynes leading to Z-selective vinyl sulfides can be achieved via Cu,9 Pd10 or Cs11 catalysis, but requires one or more additives for the transformation to take place. In such a scenario, development of a mild, rapid and stereoselective procedure, particularly in absence of any catalyst and/or additives will provide an excellent alternative to obtain vinyl sulfides.Most of the reported procedures on the hydrothiolation reaction of terminal alkynes to afford vinyl sulfides have focused on various thiols like aromatic, alkyl and benzyl thiols.5–11 Conversely, report's regarding the participation of heterocyclic thiols in hydrothiolation reaction of terminal alkynes is significantly lagging behind. In this regard, we have recently developed an indium catalyzed hydrothiolation of terminal alkynes with heterocyclic thiols to obtain vinyl sulfides12 (path a, Scheme 1) via the more challenging Markovnikov mode of addition. In extension to this finding we shifted our emphasis toward the reaction between heterocyclic thiols and electron-deficient terminal alkynes (path b, Scheme 1).
In the present study, we show that Z-selective vinyl sulfides can be generated in a simple and efficient way by reacting heterocyclic thiols with activated terminal alkynes inside a microwave reactor in absence of any catalyst or additive. The methodology is also found to be successful with aromatic thiols, albeit with a lower degree of selectivity. Over the last decade, microwave has emerged as a new and alternative mode of synthesis known as microwave assisted organic synthesis (MAOS).13 The popularity of MAOS technique among synthetic chemists can be attributed to its ability to address both environmental and economic aspects of synthesis.14
Results and discussion
Our initial objective was to develop stereoselective anti-Markovnikov addition of heterocyclic thiols across terminal alkynes as this has not been achieved so far. Preliminary studies were carried out by reacting 2-mercaptobenzothiazole 1a and ethyl propiolate 2a as model substrates. When 2-mercaptobenzothiazole 1a and ethyl propiolate 2a were stirred in water at room temperature, formation of Z-ethyl-3-(benzothiazol-2-ylthio) acrylate 3aa was observed with absolute stereoselectively in 50% yield. The biological significance of the product6c,15 and high degree of regio- and stereoselectivity encouraged us to study the reaction further and optimization studies were consequently undertaken (see Table 1).| Entry | Solvent | Temp.* °C | Conditions** | Reaction time (h min−1) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: thiol (1 mmol), ethyl propiolate (1.3 mmol) were allowed at *specified temp and **conditions (RT = room temp., CH = conventional heating and MW = microwave heating).b Isolated yield. | |||||
| 1 | Water | 30 | RT | 2 h | 50 |
| 2 | Methanol | 30 | RT | 4 h | 55 |
| 3 | Water | 100 | CH | 40 min | 62 |
| 4 | Solventless | 100 | MW | 8 min | Trace |
| 5 | Water | 100 | MW | 8 min | 72 |
| 6 | Methanol | 100 | MW | 8 min | 90 |
| 7 | Methanol | 100 | MW | 4 min | 70 |
| 8 | Methanol | 70 | MW | 15 min | 62 |
| 9 | Ethanol | 100 | MW | 8 min | 74 |
| 10 | Acetonitrile | 100 | MW | 8 min | 64 |
Optimization of the model reaction revealed that the yield could be increased to 90% (entry 6, Table 1) under much reduced time when the reactants are irradiated in methanol inside a microwave reactor at 500 W for 8 min. The reaction was clean and formation of any other side products was not detected. Other polar solvents such as water, ethanol or acetonitrile are also found to be suitable for the reaction (entries 5, 9 and 10, Table 1), however, they return a lower yield of the product. Using the optimized reaction conditions, the scope of the reaction was investigated by employing a number of different thiols and the results obtained are summarized in Table 2.
| a Reaction conditions: thiol (1 mmol), alkyne (1.3 mmol) were irradiated at 500 W for specified time inside a microwave reactor by using methanol (4 ml) as solvent. |
|---|
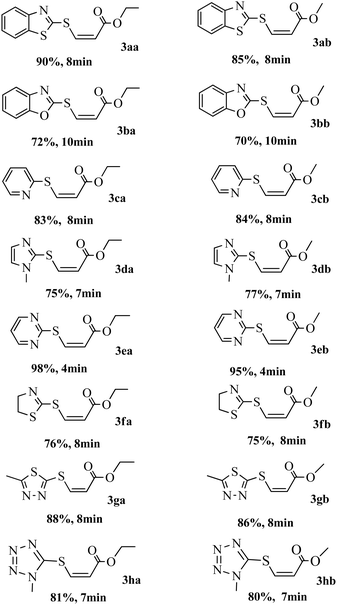 |
It was observed that a range of heterocyclic thiols participated in the reaction with absolute regio- and stereoselectivity to produce the Z-isomer of vinyl sulfide in good to excellent yields. It was encouraging to observe that thiols of various ring sizes and containing different types and number of heteroatoms reacted smoothly to give the Z-selective products without the formation of any other byproducts. Also, the reaction was complete within a very short time span of 4–10 min. Two alkynes, ethyl propiolate 2a and methyl propiolate 2b were employed to study the reactivity of the thiols. 2-Mercaptobenzoxazole 1b took 10 min to complete the reaction with 2a and 2b, while 1-methylimidazole-2-thiol 1d and 2-thiazoline-2-thiol 1f took 7 and 8 min respectively to afford the hydrothiolated products in good yield (Table 2). Excellent yields were observed with other heterocyclic thiols such as pyridine-2-thiol 1c, pyrimidine-2-thiol 1e, 1,3,4-thiadiazole-2-thiol 1g and 5-mercapto-1-methyltetrazole 1h.
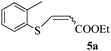
We next turned our attention to check the applicability of our method with thiols other than heterocyclic thiols. When 4-methyl thiophenol 4d was reacted with ethyl propiolate 2a at 500 W in a closed vessel inside a microwave reactor, mixture of E- and Z-vinyl sulfides was obtained with the Z-isomer as the predominant product (Table 3, entry 4).
| Entry | Thiol, 4 | Product | Time (min) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: thiol (1 mmol), ethyl propiolate (1.1 mmol) were irradiated under microwave heating till reaction is completed.b Yields of isolated products: values in parentheses indicate E/Z ratios which was determined by 1H NMR spectrum of the crude product. | ||||
| 1 |  |
 |
4 | 84 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
| 2 |  |
 |
4 | 80 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) 6) |
| 3 |  |
 |
5 | 75 (trace![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99) 99) |
| 4 |  |
 |
3 | 95 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.1) 3.1) |
| 5 | 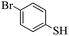 |
 |
3 | 92 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.7) 4.7) |
| 6 |  |
 |
3 | 93 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) 7) |
Although the reaction exhibited reduced selectivity, the transformation was rapid and yield was found to be excellent. Subsequently, other aromatic thiols having electron withdrawing and donating substituent were employed and similar results were obtained (Table 3, entries 1–3 and 5–6). It is noteworthy that under the current microwave reaction conditions, ortho substituted aromatic thiols like 2-methyl and 2-methoxy thiophenol also underwent the vinylation smoothly to obtain a mixture of E and Z-vinyl sulfides in short time (Table 3, entry 1 and 2). 4-Nitro thiophenol exhibited the highest Z-selectivity with only a trace amount of the E-isomer (Table 3, entry 3).
Unfortunately, aliphatic thiols did not participate in the reaction under these conditions. Efforts to activate the aliphatic thiols by elevating the temperature or altering the microwave power also failed and starting compounds were recovered.
Conclusions
In conclusion, we have developed a simple, efficient and stereoselective method for the formation of vinyl sulfides via hydrothiolation reaction between heterocyclic thiols and activated terminal alkynes under microwave irradiation. The key features of the methodology are short reaction time, good to excellent yields, absence of any side products and the achievement of 100% stereoselectivity in the case of heterocyclic thiols with no additional requirement of any catalyst and/or additives. This makes it an attractive protocol to obtain Z-vinyl sulfides.Acknowledgements
NR thanks UGC, New Delhi for the award of a research fellowship. We also thank the Director, NEIST, Jorhat, for his keen interest and constant encouragement.Notes and references
- (a) A. H. F. Lee, J. Chen, D. Liu, T. Y. C. Leung, A. S. C. Chan and T. Li, J. Am. Chem. Soc., 2002, 124, 13972–13973 CrossRef CAS PubMed; (b) A. Heynderickx, A. Samat and R. Guglielmetti, Synthesis, 2002, 1747–1751 CAS; (c) P. Johannesson, G. Lindeberg, A. Johanson, G. V. Nikiforovich, A. Gogoll, B. Synnergren, M. Le Greves, F. Nyberg, A. Karlen and A. Hallberg, J. Med. Chem., 2002, 45, 1767–1777 CrossRef CAS PubMed; (d) M. Litaudon, F. Trigalo, M.-T. Martin, F. Frappier and M. Guyot, Tetrahedron, 1994, 50, 5323–5334 CrossRef CAS.
- (a) T. Mukaiyama, K. Kamio, S. Kobayashi and H. Takei, Bull. Chem. Soc. Jpn., 1972, 4, 193–202 Search PubMed; (b) E. Wenkert, T. W. Ferreira and E. L. Michelotti, J. Chem. Soc., Chem. Commun., 1979, 6, 637–638 RSC; (c) A. Sabarre and J. A. Love, Org. Lett., 2008, 10, 3941–3944 CrossRef CAS PubMed; (d) T. H. Morris, E. H. Smith and R. Walsh, J. Chem. Soc., Chem. Commun., 1987, 964–965 RSC; (e) P. Magnus and D. Quagliato, J. Org. Chem., 1985, 50, 1621–1626 CrossRef CAS.
- R. D. Miller and R. Hassig, Tetrahedron Lett., 1985, 26, 2395–2398 CrossRef CAS.
- B. M. Trost and A. C. Lavoie, J. Am. Chem. Soc., 1983, 105, 5075–5090 CrossRef CAS.
- For reviews on C–S bond formation reactions, see: (a) T. Kondo and T.-A. Mitsudo, Chem. Rev., 2000, 100, 3205–3220 CrossRef CAS PubMed; (b) F. Alonso, I. P. Beletskaya and M. Yus, Chem. Rev., 2004, 104, 3079–3159 CrossRef CAS PubMed.
- (a) H.-L. Kao and C.-F. Lee, Org. Lett., 2011, 13, 5204–5207 CrossRef CAS PubMed; (b) M. S. Kabir, M. Lorenz, M. L. Van Linn, O. A. Namjoshi, S. Ara and J. M. Cook, J. Org. Chem., 2010, 75, 3626–3643 CrossRef CAS PubMed; (c) M. S. Kabir, O. A. Namjoshi, R. Verma, R. Polanowski, S. M. Krueger, D. Sherman, M. A. Rott, W. R. Schwan, A. Monte and J. M. Cook, Bioorg. Med. Chem., 2010, 18, 4178–4186 CrossRef CAS PubMed; (d) C. G. Bates, P. Saejueng, M. Q. Doherty and D. Venkataraman, Org. Lett., 2004, 6, 5005–5008 CrossRef CAS PubMed.
- C.-M. Chu, Z. Tu, P. Wu, C.-C. Wang, J.-T. Liu, C.-W. Kuo, Y.-H. Shin and C.-F. Yao, Tetrahedron, 2009, 65, 3878–3885 CrossRef CAS PubMed.
- For some selected examples of hydrothiolation of terminal alkynes, see: (a) Y. Yang and R. M. Rioux, Chem. Commun., 2011, 47, 6557–6559 RSC; (b) Z. Jin, B. Xu and G. B. Hammond, Eur. J. Org. Chem., 2010, 168–173 CrossRef CAS; (c) Y. Sarrafi, M. Sadatshahabi, K. Alimohammadi and M. Tajbakhsh, Green Chem., 2011, 13, 2851–2858 RSC; (d) M. Minozzi, A. Monesi, D. Nanni, P. Spagnolo, N. Marchetti and A. Massi, J. Org. Chem., 2011, 76, 450–459 CrossRef CAS PubMed; (e) M. S. Silva, R. G. Lara, J. M. Marczewski, R. G. Jacob, E. J. Lenardao and G. Perin, Tetrahedron Lett., 2008, 49, 1927–1930 CrossRef CAS PubMed; (f) S. Shoai, P. Bichler, B. Kang, H. Buckley and J. A. Love, Organometallics, 2007, 26, 5778–5781 CrossRef CAS; (g) N. A. Randive, V. Kumar and V. A. Nair, Monatsh. Chem., 2010, 141, 1329–1332 CrossRef CAS PubMed; (h) R. Sridhar, K. Surendra, N. Srilakshmi Krishnaveni, B. Srinivas and K. Rama Rao, Synlett, 2006, 3495–3497 CAS.
- (a) S. Ranjit, Z. Duan, P. Zhang and X. Liu, Org. Lett., 2010, 12, 4134–4136 CrossRef CAS PubMed; (b) Z.-L. Wang, R.-Y. Tang, P.-S. Luo, C.-L. Deng, P. Zhong and J.-H. Li, Tetrahedron, 2008, 64, 10670–10675 CrossRef CAS PubMed.
- A. Kondoh, H. Yorimitsu and K. Oshima, Org. Lett., 2007, 9, 1383–1385 CrossRef CAS PubMed.
- A. Kondoh, K. Takami, H. Yorimitsu and K. Oshima, J. Org. Chem., 2005, 70, 6468–6473 CrossRef CAS PubMed.
- R. Sarma, N. Rajesh and D. Prajapati, Chem. Commun., 2012, 48, 4014–4016 RSC.
- For reviews on Microwave-assisted organic synthesis, see: (a) Microwaves in Organic Synthesis, ed. A. Loupy, Wiley-VCH, Weinheim, Germany, 2nd edn, 2006 Search PubMed; (b) C. O. Kappe, Angew. Chem., Int. Ed., 2004, 43, 6250–6284 CrossRef CAS PubMed; (c) J. D. Moseley and C. O. Kappe, Green Chem., 2011, 13, 794–806 RSC; (d) P. Lidstrom, J. Tierney, B. Wathey and J. Westman, Tetrahedron, 2001, 57, 9225–9283 CrossRef CAS.
- (a) H. H. Nguyen and M. J. Kurth, Org. Lett., 2013, 15, 362–365 CrossRef CAS PubMed; (b) S. Castro, J. J. Fernandez, R. Vicente, F. J. Fananas and F. Rodriguez, Chem. Commun., 2012, 48, 9089–9091 RSC; (c) A. Porcheddu, R. Cadoni and L. De Luca, Org. Biomol. Chem., 2011, 9, 7539–7546 RSC; (d) K. Gormer, H. Waldmann and G. Triola, J. Org. Chem., 2010, 75, 1811–1813 CrossRef PubMed.
- M. S. Kabir, M. Lorenz, O. A. Namjoshi and J. M. Cook, Org. Lett., 2010, 12, 464–467 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra47417h |
| This journal is © The Royal Society of Chemistry 2014 |



