Rod-like NaNbO3: mechanisms for stable solvothermal synthesis, temperature-mediated phase transitions and morphological evolution†
Qilin Guab,
Kongjun Zhu*a,
Jinsong Liub,
Pengcheng Liuab,
Yang Caoc and
Jinhao Qiua
aState Key Laboratory of Mechanics and Control of Mechanical Structures, College of Aerospace Engineering, Nanjing University of Aeronautics and Astronautics, Nanjing, 210016, P. R. China. E-mail: kjzhu@nuaa.edu.cn
bCollege of Materials Science and Engineering, Nanjing University of Aeronautics and Astronautics, Nanjing 210016, P. R. China
cFrontier Research Institute for Interdisciplinary Sciences, Tohoku University, Sendai, Miyagi 982, Japan
First published on 13th March 2014
Abstract
One dimensional (1D) NaNbO3 powders have attracted increasing attention for their excellent photo-catalytic and piezoelectric properties, and the stable, moderate and low-energy synthesis of the targets is highly desirable. Herein, a facial solvothermal strategy is adapted to synthesize the one-dimensional (1D) rod-like precursors Na7(H3O)Nb6O19·14H2O by using isopropanol as the reaction medium. When the precursor is subjected to post-heating treatments, rod-like orthorhombic as well as approximate ellipsoid-like monoclinic NaNbO3 powders are obtained. Corresponding mechanisms for the stable solvothermal synthesis, morphological evolution and phase transition are further proposed and discussed.
1. Introduction
Sodium niobates (NaNbO3), which are indispensable members of the alkaline niobates, have emerged as vital functional materials with many technological and scientific applications,1,2 such as energy recovery, intelligent sensors, health monitoring, acoustic transducers, dielectric waveguides, holographic data storage, and piezoelectric actuators, because of their excellent nonlinear optical,3 ferroelectric,4 piezoelectric,5,6 pyro-electric,7 and photo-catalytic8 properties as well as their environmental friendly advantages.NaNbO3 has rich phase structures, such as orthorhombic, tetragonal, and cubic, among others.9–11 As such, explorations of the applications of some unusual phases, e.g., rhombohedral and monoclinic, are of great significance.12,13 Among the past decades, NaNbO3 with diversiform morphologies, including cubes, rods, wires, dandelions, and octahedrons, have be synthesized through various synthetic strategies and processing parameters.14 Thanks to their orientation and high aspect ratio value, one-dimensional (1D) nanowires/rods of NaNbO3 have outstanding properties both in photo-catalysts15 and piezoelectric nano-generators.16 To the best of our knowledge, rod-like NaNbO3 has been obtained through molten-salt reaction via ion-exchange using K2Nb8O21 nanowires as starting templates.17 Furthermore, NaNbO3 nanowires can be achieved by annealing the hydrothermally synthesized Na2Nb2O6 nanowires.18–24 However, both synthesis strategies have intrinsic disadvantages. The molten-salt reaction is unsuitable for large-scale synthesis given its high energy consumption and safety hazards, while the mass production of NaNbO3 nanowires is infeasible because the formation of Na2Nb2O6 nanowire requires highly alkaline conditions (10–15 M) and is sensitive to process parameters, such as the reaction temperature and time. Up to now, the controllable, moderate and low-energy synthesis of 1D NaNbO3 powders is still a meaningful project with challenges.
Previous studies25–29 indicated that addition of IPA can effectively reduce the required alkaline concentration of the niobate reaction system. Water is also reported to be unsuitable for the synthesis of many inorganic metal oxides in crystalline states.30 Moreover, solvothermal has been proved to be an advantaged approach to synthesize kinds of inorganic materials,31,32 since organic solvents with better transport properties can accelerate chemical reactions, especially when reaction temperature is approximate to or above its critical point.33,34 Limited solubility of inorganic reactants in solvents which can control the reaction level is another significant superiority. Here, we chose IPA as reaction medium to synthesize the 1D rod-like precursor Na7(H3O)Nb6O19·14H2O, and 1D rod-like orthorhombic as well as ellipsoid-like monoclinic NaNbO3 powders are successfully obtained by heat treatments. And possible mechanisms for temperature-induced phase transition and morphological evolution are further identified.
2. Experimental procedure
2.1. Synthesis and preparation
All chemicals were of analytical grade and used as received without further purification. In a typical solvothermal procedure for synthesizing the rod-like precursor Na7(H3O)Nb6O19·14H2O, 2.083 g of sodium hydroxide (NaOH, 96% min, Sinopharm Chemical Reagent Co., Ltd.) was dissolved into 50 mL of IPA (C3H8OH, 99.7% min, Sinopharm Chemical Reagent Co., Ltd.) through vigorous magnetic stirring. Niobium oxide (Nb2O5, 99.5% min, Sinopharm Chemical Reagent Co., Ltd.) was added slowly into this solution after 30 min of stirring. The mixture was then stirred vigorously at room temperature for another 30 min, transferred to a Teflon-lined autoclave (70 mL), and heated to 230 °C. After 16 h of heating, the mixture was naturally cooled to room temperature. The product was rinsed with de-ionized water and anhydrous alcohol, and precipitated with centrifugation for 5 min at 3000 rpm to yield a white, rod-like precursor powder. Rinsing was repeated thrice to remove excess ions from the final product, and the precursor was dried at 80 °C overnight.The as-obtained precursor was subjected to heat treatments in air at 300–800 °C for 4 h to obtain the final products. The NaOH + IPA solution without Nb2O5 was stirred continuously both at room temperature and during heating, and a pH meter (EUTECH 510) recorded the pH of the mixture every 10 min to evaluate the solubility of NaOH in IPA solvent.
2.2. Characterization
The crystal structure of the as-prepared sample was characterized by a Bruker X-ray diffractometer XRD, Bruker D8 Advance, Germany with a Cu Kα radiation (40 kV, 40 mA) source (λ = 0.154178 nm) at a scanning rate of 10° min−1 in the 2θ range of 5°–60°. To analyze the thermal behavior of as-synthesized precursor, thermo-gravimetric analysis and differential scanning calorimetry (TGA-DSC, NETZSCH-STA 409PC, German) were conducted in Ar atmosphere at a heating rate of 10 °C min−1 from room temperature to 900 °C. Using a 514.5 nm laser, Raman spectra were measured by a confocal laser micro-Raman spectroscopy system (Raman, LABRAM HR800) within the range of 100–1000 cm−1. The morphology and microstructure of as-synthesized samples were observed using a Hitachi S-4800 field emission scanning electron microscope (FE-SEM, Hitachi S-4800, Japan) and a transmission electron microscope (TEM; JEOL JEM-2100, Japan).3. Results and discussion
3.1. Synthesis of Na7(H3O)Nb6O19·14H2O
Fig. 1 shows the crystalline structure of as-obtained product determined by XRD pattern. The sample is identified as orthorhombic-phase Na7(H3O)Nb6O19·14H2O (JCPDS Standard Card no.: 84-0188; space group: Pmnn; lattice parameters: a = 10.072 Å, b = 12.148 Å, c = 12.722 Å). This result reveals an important fact that Na7(H3O)Nb6O19·14H2O can be formed at a relatively low alkalinity of 1 M, owing to the unique characteristics of IPA, including polarity, viscosity, surface tension, and boiling point, etc.35 Conventionally, synthesizing the same products via hydrothermal route requires a higher alkalinity ranging from 10 M to 15 M, and the formation process is difficult to control.36–38 The IPA-based solvothermal strategy provides a facile, controllable, and moderate process for obtaining intermediate Na7(H3O)Nb6O19·14H2O and can be extended to the selective synthesis of the other multiphase materials. | ||
| Fig. 1 Powder XRD patterns of as-synthesized precursor using organic solvent isopropanol as reaction medium (230 °C, 16 h). | ||
In a previous study, alkaline niobate powders were synthesized in solution via a dissolution–precipitation process,39 and the pertinent reaction processes can be written as follows:40
| Nb2O5 + 8OH− → Nb6O198− + H2O | (1) |
| Nb6O198− + 34OH− → NbO67− + 17H2O | (2) |
| A+ + NbO67− + 3H2O → ANbO3 + 6OH− | (3) |
A+ in reaction (3) refers to alkali ions, such as Na+ and K+. Reactions (1) and (2) show that the alkaline concentration required for the formation of NbO67− anions from Nb6O198− should be higher than that necessary for the dissolution of Nb2O5. Actually, the pH value of the designed 1 M NaOH + IPA solution reaches 13.2 through vigorous stirring and heating (Fig. 2). The pH value range is beneficial for the stable existence of Nb6O198−.41 Therefore, the actual OH− concentration is limited, and the reaction process can only proceed to step (1), while step (2) requiring higher alkaline concentration would not occur. This may be a necessary factor accountable for the stable synthesis of Na7(H3O)Nb6O19·14H2O in organic solvent IPA. However, almost all the previous reported solvothermal synthesis of alkali niobates are conducted in mixture solutions of water and organic solvents, and the unique effects of organic solvents cannot work out effectively.27–29 Thus, solvothermal route using pure organic solvent as reaction medium is considered to be a promising method in synthesizing niobate and other inorganic powders.
 | ||
| Fig. 2 Solubility of NaOH in organic isopropanol solvent at room temperature and heating condition evaluated by the pH value as a function of time. Results are presented as mean ± SEM (error bar). | ||
Fig. 3 shows the DSC-TGA curves of the precursor heated from room temperature to 900 °C. Significant weight loss is observed as the temperature increased from 80 °C to 200 °C, which is attributed to the elimination of crystallization water and hydration water from Na7(H3O)Nb6O19·14H2O. With further increases of temperature, the mass loss rate slows down and ceases at about 300 °C. The weight loss observed is highly similar to that found in the thermogram of the same product prepared by the molten method, corresponding to the mass of 14.4H2O.42 As the temperature increased from 600 °C to 800 °C, a slight weight loss of ∼2% can be observed, which is attributed to the evaporation of sodium ions. The DSC plot for the decomposition of the product in Ar gas shows four peaks (blue curve in Fig. 3). The first peak at 122 °C indicates an endothermic event corresponding to the rapid release of H2O. The second peak at 414 °C presents the transformation of the precursors into the NaNbO3 phase. The remaining peaks, which are located at 585 and 856 °C, are likely related to the phase transition of NaNbO3. This transition will be clarified in the phase structure analysis in the next section.
3.2. Phase structure analysis of the powder after annealing
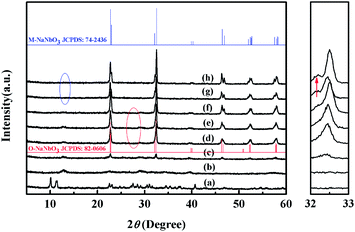
Annealing treatments from 300 °C to 800 °C were conducted based on the DSC-TGA results. Fig. 4 shows the XRD patterns of the powders obtained after annealing. Fig. 4(a) displays the XRD pattern of as-prepared Na7(H3O)Nb6O19·14H2O for comparison. After heating treatment at 300 °C for 4 h, the strong characteristic diffraction peaks [(011)/(101)/(110)] of Na7(H3O)Nb6O19·14H2O disappear, and no other diffraction peaks is observed [Fig. 4(b)]. When the annealing temperature increased to 350 °C, two diffraction peaks at 2θ of 22° and 32° are observed, as shown in Fig. 4(c). As the temperature continuously increased to 400 °C [Fig. 4(d)], the intensity of the diffraction peaks is significantly enhanced and these peaks can be indexed to the orthorhombic perovskite structure of NaNbO3 (JCPDS Standard Card no.: 82-0606, space group: P21ma, lattice parameters: a = 5.569 Å, b = 7.790 Å, c = 5.518 Å). During annealing at 400 °C, the transient Na7(H3O)Nb6O19·14H2O phase may be expected to transform into the orthorhombic NaNbO3 (O-NaNbO3). A similar transformation has been observed upon formation of KxNa1−xNbO3 nanorods.43 Fig. 4(d)–(f) demonstrate that when the temperature rises to 600 °C, the O-NaNbO3 is maintained and the intensity of the diffraction peaks is further increased. Moreover, the diffraction peaks shift slightly to a higher diffraction angle. The crystal parameters slightly decrease based on the Bragg equation (2d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = λ). As the temperature increased from 600 °C to 700 °C, the diffraction peaks shift to a lower angle, which is attributed to the transformation of the phase structure of the products, as evidenced by appearance of a new peak [marked by a red arrow in the magnified Fig. 4(g)]. This peak is attributed to monoclinic NaNbO3 (M-NaNbO3, JCPDS Standard Card no.: 74-2436; space group: P2/m; lattice parameters: a = 3.909 Å, b = 3.871 Å, c = 3.909 Å, β = 90.53°). Moreover, by increasing the temperature to 800 °C, as shown in Fig. 3(h), the diffraction peaks shift to the higher angles and their intensities are enhanced. The strong dependence of the phase transformations of niobium oxide on the heat treatments has also been evidenced by Raman spectra as shown in Fig. S1.†
θ = λ). As the temperature increased from 600 °C to 700 °C, the diffraction peaks shift to a lower angle, which is attributed to the transformation of the phase structure of the products, as evidenced by appearance of a new peak [marked by a red arrow in the magnified Fig. 4(g)]. This peak is attributed to monoclinic NaNbO3 (M-NaNbO3, JCPDS Standard Card no.: 74-2436; space group: P2/m; lattice parameters: a = 3.909 Å, b = 3.871 Å, c = 3.909 Å, β = 90.53°). Moreover, by increasing the temperature to 800 °C, as shown in Fig. 3(h), the diffraction peaks shift to the higher angles and their intensities are enhanced. The strong dependence of the phase transformations of niobium oxide on the heat treatments has also been evidenced by Raman spectra as shown in Fig. S1.†
Table 1 lists the lattice parameters of all of the samples. An increase in temperature gives rise to a gradual decrease among the lattice parameters of both orthorhombic and monoclinic NaNbO3, which is in accordance with the shifting of peaks in the XRD patterns [Fig. 4(d)–(h)]. The evaporation of alkali metal elements A (A = Li, Na, K) at high temperatures can shift the diffraction peaks of alkali niobates to a higher angle (including KNbO3, NaNbO3, LiNbO3, and their solid solutions).44,45 However, the temperature-dependent shift of the diffraction peaks below 600 °C has never been reported. Thus, we propose a possible mechanism based on the formation process of NaNbO3 to explain this shift.
| Temperature (°C) | Material | Crystal system | Space group | Lattice parameters | |||||
|---|---|---|---|---|---|---|---|---|---|
| a (Å) | b (Å) | c (Å) | α (°) | β (°) | γ (°) | ||||
| Precursor | Na7(H3O)Nb6O19·14H2O | Orthorhombic | Pmnn(58) | 10.074(2) | 12.136(1) | 12.7334 | 90 | 90 | 90 |
| 400 | NaNbO3 | Orthorhombic | Pm21(26) | 5.536(8) | 7.814(4) | 5.519(1) | 90 | 90 | 90 |
| 500 | NaNbO3 | Orthorhombic | Pm21(26) | 5.518(7) | 7.804(8) | 5.498(1) | 90 | 90 | 90 |
| 600 | NaNbO3 | Orthorhombic | Pm21(26) | 5.478(8) | 7.793(6) | 5.493(2) | 90 | 90 | 90 |
| 700 | NaNbO3 | Monoclinic | P2/m(10) | 3.929(3) | 3.879(2) | 3.919(9) | 90 | 90.6 | 90 |
| 800 | NaNbO3 | Monoclinic | P2/m(10) | 3.913(3) | 3.870(3) | 3.914(1) | 90 | 90.57 | 90 |
In solvothermally synthesized Na7(H3O)Nb6O19·14H2O, the position of Na may be partially occupied by H3O+ because the ionic radius of Na+ (∼1.54 Å) is similar to that of H3O+ (∼1.35 Å).46 Through dehydration and decomposition during heating treatment, sodium vacancies are produced after the elimination of crystal water. These sodium vacancies are unstable, and the unit cell will shrink to decrease the volume of the vacancies through adequate driving forces from the external environment. An increase in temperature aggravates the level of shrinkage, as reflected by the shift of diffraction peaks to higher angles.
Compositional analysis was conducted by energy-dispersive X-ray spectroscopy (EDS), and Fig. 5 shows the ratio of Na/Nb as a function of temperature. The ratio of Na/Nb at 400 and 600 °C changed vanishingly (Δ1), which confirms that the shrinkage of lattice parameters is not mainly induced by the evaporation of alkali metal elements. As the temperature increased from 600 °C to 800 °C, the ratio of Na/Nb decreases sharply (Δ2, 13.44 times of Δ1), disclosing that the evident decrease in Na resulted from evaporation at such temperatures shifts the diffraction of M-NaNbO3 to higher angles [Fig. 4(h)] and changes O-NaNbO3 [Fig. 4(f)] to M-NaNbO3 [Fig. 4(g)]. From XRD (Fig. 4) and EDS (Fig. S2†) results, we can conclude that the present systems are of high purity. Temperature-mediated crystal structure transformation is a complex process involving dehydration, decomposition, and element volatilization. Understanding this reaction mechanism is essential to prepare varying crystals of the desirable phases.
 | ||
| Fig. 5 The molar ratio value of Na/Nb of the samples obtained at different calcination temperatures. | ||
3.3. Morphology evolutions of the powders after annealing
The morphology, grain size, and crystal structure of the samples are investigated by TEM and SAED (Fig. 6). As observed, individual Na7(H3O)Nb6O19·14H2O has a rod-like shape with a width and length of 193.2 ± 1.3 and 1193.8 ± 53.1 nm, respectively. Compared with that described in previous reports, this precursor displays a square-shaped cross section [marked by a red arrow in Fig. 6(a)], and the surfaces of the rod are smoother and more compact. It should be pointed out that Na7(H3O)Nb6O19·14H2O is not particularly stable, as it prefers being decomposed and transformed into poor crystallites or amorphous states upon heating treatment [Fig. 4(b)]. Such decomposition and transformation processes might happen by irradiation of a high-energy electron beam during TEM characterization. Therefore, it is reasonable that the SAED patterns of Na7(H3O)Nb6O19·14H2O shows an amorphous facula [Fig. 6(d)]. As shown in Fig. 6(b), O-NaNbO3 also possesses the rod-like shape. Though its morphology is similar to the Na7(H3O)Nb6O19·14H2O, the microstructure of O-NaNbO3 becomes looser, more rough, and more irregular, with the increased width and length of 305.1 ± 15.5 and 1505.6 ± 90.0 nm, respectively. As for M-NaNbO3, its morphologies drastically changed from the original rods into near-ellipsoid shapes of 512.3 ± 16.2 nm in width and 548.6 ± 42.9 nm length [Fig. 6(c)]. The two opposite edges of the ellipsoid appear to be parallel to each other whereas the other two present apparent radians, which should be related to the square-shaped rod-like nature of the Na7(H3O)Nb6O19·14H2O precursor. As revealed by the SAED patterns in Fig. 6(e) and (f), both M-NaNbO3 and O-NaNbO3 present fine crystallites. Additionally, a more detailed statistical analysis reporting the size distribution of the powders is shown in Fig. S3.† The size distribution results are obtained from several TEM images, and Fig. S3(a), (d) and (g)† are the representative TEM image of samples prepared solvothermally and treated at 400 °C and 600 °C, respectively. After the heat treatment at 400 °C [Fig. S3(e) and (f)†], the average size of powders slightly increases both in length and width compared to the solvothermal prepared samples [Fig. S3(b) and (c)†].Apparent differences in the microstructure, shape, and grain size of each phase motivated deep studies on the morphological evolution of the products. Fig. 8 shows the FE-SEM images of the products obtained as a function of the annealing temperature. The images obtained highlight the importance of temperature on the morphological evolution of the products. Fig. 7(b), for example, shows that the main shape and size of the sample obtained after heating at 300 °C for 4 h are not significantly different from those of the Na7(H3O)Nb6O19·14H2O precursors [Fig. 7(a)]. However, a small amount of white particles appeared on the surface of the rods. As the temperature increased to 350 °C [Fig. 7(c)] and 400 °C [Fig. 7(d)], the basic rod structures become loosened and grainy, while white particles on the surfaces of the structures disappears. As previously reported,42 the transition from Na7(H3O)Nb6O19·14H2O to NaNbO3 would generate a certain amount of NaOH (melting point: 318 °C). Hence, the white particles here are inferred to be NaOH, and the disappearance at 350 °C is resulted from their molten.
 | ||
| Fig. 7 Temperature-induced morphology evolution observed by FE-SEM: (a) as-synthesized Na7(H3O)Nb6O19·14H2O; (b) 300 °C, (c) 350 °C, (d) 400 °C, (e) 500 °C, (f) 600 °C, (g) 700 °C and (h) 800 °C. | ||
When the annealing temperature is increased to 500 and 600 °C [Fig. 7(e) and (f)], the rod-like shapes are still maintained as the grains grow into the average sizes of 180 and 400 nm, respectively, at the expense of small grains. It is notable that some cavities are generated in the rods, and higher temperatures slightly collapse the rod-like shapes to form dispersive particles [Fig. 7(f)]. Therefore, the binding force between the particles is decreased as the temperature increasing. Nano-scalar particles can be obtained through ultrasonic treatment of the loose rods; such treatment provides a novel method for preparation of nanoparticles (NPs) from destruction of micro-rods (MRs). The size of the NPs may be controlled by tailoring the aspect ratio of the MRs. The work on this topic is currently underway.
Fig. 7(g) and (h) show FE-SEM images of the samples obtained after thermal treatments above 700 °C. No trace of the rod-like structure is observed in these images, and all of the particles appear as irregular ellipsoids. By increasing the temperature from 700 °C to 800 °C, the ellipsoid-like grains grow rapidly from ∼500 nm to ∼1 μm with a broad size distribution.
3.4. Mechanisms of morphological evolution
Fig. 8 schematically illustrates the crystal structure and morphology transformation during subsequent calcination of Na7(H3O)Nb6O19·14H2O. When the temperature is increased to 400 °C, as-synthesized Na7(H3O)Nb6O19·14H2O is converted into the orthorhombic-structured NaNbO3 without changing its basic rod-like shape. Given that the transformation process involves (a) the dehydration reaction from Na7(H3O)Nb6O19·14H2O to Na7[HNb6O19] and (b) the decomposition reaction from Na7[HNb6O19] to O-NaNbO3 and NaOH, the structure of the rod-like O-NaNbO3 becomes looser as it is composed of a large amount of tiny grains with high surface energy. Similar rough interconnected crystalline structures have been observed in solvothermal synthesized metal oxides (including TiO2, SnO2, In2O3 and PbO).47 The changes in structure and morphology are considered to be originated from the phase transformation (for example, from anatase to rutile in TiO2) as well as the growth of crystallites at higher annealing temperatures. However, XRD and SEM results indicate that, the morphology in our work can be derived from the initial formation of NaNbO3 from decomposition process and the growth of crystallites. As temperature increased to 700 °C, phase transformation from orthorhombic to monoclinic occurs, and the crystallites growth process continues. The results indicate that the phase transformation from orthorhombic to monoclinic is oriented rearrangement process, and cannot bring evident changes in morphology. Likewise, the rod/wire-like O-NaNbO3 obtained from the intermediate phase Na2Nb2O6·nH2O has a surface that is as smooth as that of Na2Nb2O6·nH2O.18–24 The evaporation of alkali ions at high temperature is inevitable, especially at temperatures above 600 °C (Fig. 4); this evaporation accounts for the phase transformation from O-NaNbO3 to M-NaNbO3. | ||
| Fig. 8 Schematic illustration of phase transitions and morphology evolutions induced by various calcination temperatures. | ||
4. Conclusions
A facile and stable solvothermal process is developed for synthesis of rod-like Na7(H3O)Nb6O19·14H2O, and IPA is found to be a favorable reaction medium. The Na7(H3O)Nb6O19·14H2O precursor could be further transformed to NaNbO3 with various crystal structures, shapes, and grain sizes by simple thermal treatments. Gradual increase of the heating temperature gives rise to transformation from rod-like Na7(H3O)Nb6O19·14H2O to rod-like Na7[HNb6O19], rod-like O-NaNbO3, and finally approximately ellipsoid-like M-NaNbO3. The elimination H3O+ derived from solvothermal synthesized precursors gives rise to the cell unites shrinkage at low temperature. Sodium ion evaporation accounts for the phase transformation from O-NaNbO3 to M-NaNbO3, while decomposition from Na7(H3O)Nb6O19·14H2O to NaNbO3 and NaOH leads to the formation of loose, rod-like structures and eventual collapse of these rods into ellipsoid particles. This investigation on solvothermal synthesis, thermal-induced structures, and morphological transformations has been offering the foundation for property study and functional application of alkali niobates with desirable phase structures, morphologies, and sizes.Acknowledgements
This work was supported by the National Nature Science Foundation of China (NSFC no. 51172108), A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.References
- W. Zeng, X. M. Tao, S. Chen, S. M. Shang, H. L. W. Chan and S. H. Choy, Energy Environ. Sci., 2013, 6, 2631–2638 CAS.
- M. Blomqvist, S. Khartsev, A. Grishin, A. P. etraru and C. Buchal, Appl. Phys. Lett., 2003, 82, 439 CrossRef CAS.
- F. Dutto, C. Raillon, K. Schenk and A. Radenovic, Nano Lett., 2011, 11(6), 2517–2521 CrossRef CAS PubMed.
- C. L. Yan, L. Nikolova, A. Dadvand, C. Harnagea, A. Sarkissian, D. F. Perepichka, D. F. Xue and F. Rosei, Adv. Mater., 2010, 22(15), 1741–1745 CrossRef CAS PubMed.
- J. H. Jung, M. Lee, J. I. Hong, Y. Ding, C. Y. Chen, L. J. Chou and Z. L. Wang, ACS Nano, 2011, 5(12), 10041–10046 CrossRef CAS PubMed.
- Y. Saito, H. Takao, T. Tani, T. Nonoyama, K. Takatori, T. Homma, T. Nagaya and M. Nakamura, Nature, 2004, 432, 84–87 CrossRef CAS PubMed.
- S. T. Lau, C. H. Cheng, S. H. Choy, D. M. Lin, K. W. Kwok and H. L. W. Chan, J. Appl. Phys., 2008, 103, 104105 CrossRef.
- S. F. Chen, L. Ji, W. M. Tang and X. L. Fu, Dalton Trans., 2013, 42, 10759–10768 RSC.
- S. K. Mishra, N. Choudhury, S. L. Chaplot, P. S. R. Krishna and R. Mittal, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 024110 CrossRef.
- P. Li, S. X. Ouyang, Y. J. Zhang, T. Kako and J. H. Ye, J. Mater. Chem. A, 2013, 1, 1185–1191 CAS.
- T. T. Zhang, K. Zhao, J. G. Yu, J. Jin, Y. Qi, H. Q. Li, X. J. Hou and G. Liu, Nanoscale, 2013, 5, 8375–8383 RSC.
- D. R. Modeshia, R. J. Darton, S. E. Ashbrook and R. I. Walton, Chem. Commun., 2009, 68–70 RSC.
- K. E. Johnston, C. C. Tang, J. E. Parker, K. S. Knight, P. Lightfoot and S. E. Ashbrook, J. Am. Chem. Soc., 2010, 132(25), 8732–8746 CrossRef CAS PubMed.
- K. J. Zhu, Y. Cao, X. H. Wang, L. Bai, J. H. Qiu and H. L. Ji, CrystEngComm, 2012, 14, 411–416 RSC.
- J. Lv, T. Kako, Z. S. Li, Z. G. Zou and J. H. Ye, J. Phys. Chem. C, 2010, 114, 6157–6162 CAS.
- T. Y. Ke, H. A. Chen, H. S. Sheu, J. W. Yeh, H. N. Lin, C. Y. Lee and H. T. Chiu, J. Phys. Chem. C, 2008, 112, 8827–8831 CAS.
- C. Y. Xu, L. Zhen, R. Yang and Z. L. Wang, J. Am. Chem. Soc., 2007, 129, 15444–15445 CrossRef CAS PubMed.
- H. F. Shi, T. Z. Wang, J. Chen, C. Zhu, J. H. Ye and Z. G. Zou, Catal. Lett., 2011, 141, 525–530 CrossRef CAS.
- H. W. Xu, M. Nyman, T. M. Nenoff and A. Navrotsky, Chem. Mater., 2004, 16, 2034–2040 CrossRef CAS.
- J. H. Jung, C. Y. Chen, W. W. Wu, J. I. Hong, B. K. Yun, Y. S. Zhou, N. Lee, W. Jo, L. J. Chen, L. J. Chou and Z. L. Wang, J. Phys. Chem. C, 2012, 116(42), 22261–22265 CAS.
- A. Yu, J. S. Qian, L. Liu, H. Pan and X. F. Zhou, Appl. Surf. Sci., 2012, 258, 3490–3496 CrossRef CAS.
- H. F. Shi, X. K. Li, D. F. Wang, Y. P. Yuan, Z. G. Zou and J. H. Ye, Catal. Lett., 2009, 132, 205–212 CrossRef CAS.
- A. J. Paula, M. A. Zaghete, E. Longo and J. A. Varela, Eur. J. Inorg. Chem., 2008, 1300–1308 CrossRef CAS.
- L. Liu, B. Li, D. H. Yu, Y. M. Cui, X. F. Zhou and W. P. Ding, Chem. Commun., 2010, 46, 427–429 RSC.
- M. Zhang, M. Guo and Y. Zhou, Int. J. Appl. Ceram. Technol., 2011, 8(3), 591–596 CrossRef CAS.
- A. D. Handokoa and G. K. L. Goh, Green Chem., 2010, 12, 680–687 RSC.
- H. H. Gu, K. J. Zhu, X. M. Pang, B. Shao, J. H. Qiu and H. L. Ji, Ceram. Int., 2012, 38, 1807–1813 CrossRef CAS.
- L. Bai, K. J. Zhu, L. K. Su, J. H. Qiu and H. L. Ji, Mater. Lett., 2010, 64, 77–79 CrossRef CAS.
- L. K. Su, K. J. Zhu, L. Bai, J. H. Qiu and H. L. Ji, J. Alloys Compd., 2010, 493, 186–191 CrossRef CAS.
- D. Koziej, C. Floryan, R. A. Sperling, A. J. Ehrlicher, D. Issadore, R. Westervelt and D. A. Weitz, Nanoscale, 2013, 5, 5468–5475 RSC.
- G. Xu, Z. H. Tao, Y. G. Zhao, Y. F. Zhang, Z. H. Ren, G. Shen, G. R. Han and X. Wei, CrystEngComm, 2013, 15, 1439–1444 RSC.
- H. Zhu, C. Hou, Y. J. Li, G. H. Zhao, X. Liu, K. Hou and Y. F. Li, Chem. – Asian J., 2013, 8(7), 1447–1454 CrossRef CAS PubMed.
- H. H. Gu, K. J. Zhu, J. H. Qiu, H. L. Ji, Y. Cao and J. M. Jin, J. Nanosci. Nanotechnol., 2013, 13(2), 1317–1322 CrossRef CAS PubMed.
- A. R. Katrizky, D. A. Nichols and M. Siskin, Chem. Rev., 2001, 101, 837–892 CrossRef.
- F. Chen, Y. J. Zhu, X. Y. Zhao, B. Q. Lu and J. Wu, CrystEngComm, 2013, 15, 4527–4531 RSC.
- H. W. Song and W. H. Ma, Ceram. Int., 2011, 37, 877–882 CrossRef CAS.
- T. M. Alam, M. Nyman, B. R. Cherry, J. M. Segall and L. E. Lybarger, J. Am. Chem. Soc., 2004, 126(17), 5610–5620 CrossRef CAS PubMed.
- M. Tanaka and S. Fujihara, Eur. J. Inorg. Chem., 2012, 1180–1185 CrossRef CAS.
- H. Liu, C. G. Hu and Z. L. Wang, Nano Lett., 2006, 6(7), 1535–1540 CrossRef CAS PubMed.
- Y. M. Hu, H. S. Gu, Z. L. Hu, W. N. Di, Y. Yuan, J. You, W. Q. Cao, Y. Wang and H. L. W. Chan, Cryst. Growth Des., 2008, 8(3), 832–837 CAS.
- A. Goiffon, R. Granger, C. Bockel and B. Spinner, Rev. Chim. Miner., 1973, 10(3), 487–502 CAS.
- S. Yamazoe, T. Kawawaki, T. Imai and T. Wada, J. Ceram. Soc. Jpn., 2010, 118(8), 741–744 CrossRef CAS.
- H. B. Xu, M. R. Joung, J. S. Kim, S. Nahm, M. G. Kang, C. Y. Kang and S. J. Yoon, Chem. Eng. J., 2012, 211–212, 16–21 CrossRef CAS.
- W. F. Liang, D. Q. Xiao, W. J. Wu, X. H. Li, Y. Sun and J. G. Zhu, Curr. Appl. Phys., 2011, 11, S138–S142 CrossRef.
- J. Fang, X. H. Wang, Z. B. Tian, C. F. Zhong and L. T. Li, J. Am. Ceram. Soc., 2010, 93(11), 3552–3555 CrossRef CAS.
- G. Cao, R. C. Haushalter and K. G. Strohmaier, Inorg. Chem., 1993, 32(2), 127–128 CrossRef CAS.
- X. C. Jiang, Y. L. Wang, T. Herricks and Y. N. Xia, J. Mater. Chem., 2004, 14, 695–703 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra47391k |
| This journal is © The Royal Society of Chemistry 2014 |