An optimized LiNO3/DMSO electrolyte for high-performance rechargeable Li–O2 batteries†
Abstract
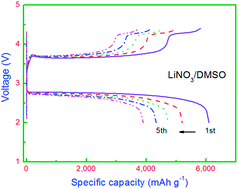
Finding stable electrolytes is essential to address the poor cycling capability of current rechargeable non-aqueous Li–O2 batteries. An optimized dimethyl sulfoxide (DMSO) based electrolyte using lithium nitrate (LiNO3) as the lithium salt has been first investigated for rechargeable Li–O2 batteries. The charge over-potential of Li–O2 batteries with LiNO3/DMSO electrolyte is 0.42 V lower than that of batteries with LiClO4/DMSO electrolyte. The Li–O2 batteries with LiNO3/DMSO electrolyte also showed excellent high C-rate performance and good cycling stability.

 Please wait while we load your content...
Please wait while we load your content...