First total synthesis of cryptosporiopsin A†
Abstract
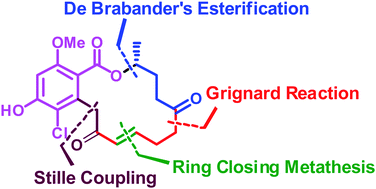
The first total synthesis of polyketide natural product cryptosporiopsin A (1) was described. It has been accomplished in 12 longest linear steps with 15.4% overall yield starting from enantiomerically pure epoxide 12 prepared by hydrolytic kinetic resolution. Other key steps were Stille coupling, Grignard reaction, De Brabander's esterification and ring closing metathesis (RCM) reaction.

 Please wait while we load your content...
Please wait while we load your content...