Copper(II)-catalyzed cascade approach for the synthesis of pyrrolo[2,1-f][1,2,4]triazine-fused isoquinolines†
Jianyang Chena,
Bo Liu*b,
Yanhong Chena,
Qian Hea and
Chunhao Yang*a
aState Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, 555 Zu Chong Zhi Road, Shanghai 201203, P.R. China. E-mail: chyang@simm.ac.cn; Fax: +86-21-50806770
bGuangdong Provincial Academy of Chinese Medical Sciences, (The Second Affiliated Hospital of Guangzhou University of Chinese Medicine), 5th Floor, Science Building, 55 Neihuanxi Road, Guangzhou Higher Education Mega Center, Guangzhou, 510006, P.R. China. E-mail: doctliu@263.net
First published on 10th February 2014
Abstract
We report herein a one-pot copper(II)-catalyzed coupling-cyclization leading to small molecules based on a novel structural motif, i.e. the pyrrolo[2,1-f][1,2,4]triazine moiety fused with an isoquinoline ring. The reaction is easy to perform in good to excellent yields with high atom economy and exhibits a broad substrate scope.
Introduction
The development of synthetic organic chemistry in pharmaceutical science provides new opportunities to synthesize new bioactive chemical entities. The transition-metal-catalyzed cascade cyclization reaction has been used as a major method to construct heterocyclic scaffolds from readily accessible organic compounds.1 In the past few years, Lewis acids (complexes of Ag, Cu, Fe, Au, Pd etc.) have emerged as powerful catalysts for the purpose. The feasibility of activating C-heteroatom bonds like C–O, C–N, C–S bond by coordination to these catalysts has led to the development of a variety of catalytic reactions involving C-heteroatom bond formation with high atom economy.2As a privileged fragment, pyrrolo[2,1-f][1,2,4]triazine skeleton is an important moiety that has been found to be present in several pharmaceutical candidates, exhibiting anticancer,3 anti-inflammatory4 activities (Fig. 1). For example, EGFR tyrosine kinase inhibitors BMS690514,5 BMS599626![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 and IGF1R inhibitor BMS754807
6 and IGF1R inhibitor BMS754807![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 all contain a pyrrolo[2,1-f][1,2,4]triazine scaffold. Therefore, the design and synthesis of novel pyrrolo[2,1-f][1,2,4]triazine scaffolds attract the attention of both organic and medicinal chemists.8
7 all contain a pyrrolo[2,1-f][1,2,4]triazine scaffold. Therefore, the design and synthesis of novel pyrrolo[2,1-f][1,2,4]triazine scaffolds attract the attention of both organic and medicinal chemists.8
Isoquinoline-fused polycyclic compounds are important structural moieties that appear in both natural products and therapeutic agents and have wide applications in pharmaceutical research (Fig. 1).9 Considerable efforts have been made to develop efficient methods for the synthesis of this class of compounds. Using cascade addition of nucleophiles and cyclization, many reports in the literature9b,10 have showed the successful synthesis of fused 1,2-dihydroisoquinolines in the presence or absence of Lewis acid catalysts like AgOTf,11 AuCl,12 or Yb(OTf)3 (ref. 13) (Scheme 1 eqn 1).
 | ||
| Scheme 1 A possible route for the construction of diverse 4bH-isoquino[2,1-a]pyrrolo[2,1-f][1,2,4]triazin-6(5H)-one. | ||
As a part of our interest in the construction of heterocyclic scaffolds using substituted N-amino pyrroles as building blocks to expand medicinal chemistry space,8a,14 in this paper we hypothesized that the condensation reaction of 2-alkynylbenzaldehydes and N-amino pyrrole-2-carboxyamide would provide the corresponding imine intermediate, which would follows cascade addition of nucleophiles and cyclization to afford fused isoquinolines in the presence of appropriate Lewis acids (Scheme 1 eqn (2)).
Results and discussion
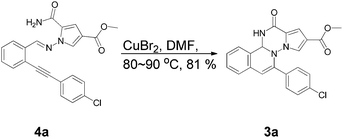
In order to explore this proposal, methyl 1-amino-5-carbamoyl-1H-pyrrole-3-carboxylate (1a) was treated with 1.2 equiv. of 2-((4-chlorophenyl)ethynyl)benzaldehyde (2a) in the presence of a variety of transition metal catalysts and solvents. The results were summarized in Table 1. When the reaction was treated by AuCl (0.1 equiv.) in the presence of 4 Å MS in dichloroethane (DCE) at room temperature, the expected product 3a was not obtained, but imine 4a was isolated in 59% yield (Table 1, entry 1). Elevating the temperature to 80–90 °C gave the product 3a in 30% yield and the imine 4a in 20% yield (Table 1, entry 2). When gold(I) chloride was replaced by more cheaper copper catalyst Cu(OTf)2, the same yield was obtained (Table 1, entry 3 vs. entry 2). In order to improve the solubility of reactants, dimethyl formamide (DMF) was employed as the solvent and the product 3a was obtained in 65% yield (Table 1, entry 4). Then other catalysts such as CuCl2·2H2O and CuBr2 were screened. 3a was obtained in 72% and 83% yields, respectively (Table 1, entries 5 and 6). Without the catalyst the target product 3a was not afforded, with a result of imine 4a obtained in 73% yield (Table 1, entry 12). However, CuCl, Cu(NO3)2·3H2O, anhydrous FeCl3 did not make this transformation proceed under the conditions (Table 1, entries 7–9), and only the imine 4a was obtained. We speculate the transformation may proceed through the imine intermediate. So we conducted an experiment using imine 4a and CuBr2 in DMF at 80–90 °C to prove the proposition. Fortunately the imine can transform to the target compound in 81% yield (shown in Scheme 2). However, addition of base and ligand failed to give the desired product 3a (Table 1, entries 10 and 11). Finally we also investigated the effect of reaction time. The yield was slightly lowered when monitored the progress in 2 hours, and the yield did not change when extending reaction time to 7 hours (Table 1, entries 13 and 14). The compound 3a was characterized by the 1H NMR, 13C NMR and DEPT spectra analysis.| Entry | Catalyst | Additive | Solvent | Temp./°C | Yield of 3ab | 4ac |
|---|---|---|---|---|---|---|
| a Reaction condition: 1a (0.2 mmol), 2a (0.24 mmol), catalyst (0.02 mmol), solvent (3 ml), 4 h.b Yield isolated by column chromatograph.c Yield isolated by column chromatograph.d Phen (0.04 mmol), DBU (0.4 mmol) was added.e Reaction time for 2 h.f Reaction time for 7 h. r.t.: room temperature. Phen: 1,10-phenanthroline. DBU:1,8-diazabicyclo[5.4.0]undec-7-ene. n.d. = not detected. | ||||||
| 1 | AuCl | 4 Å MS | DCE | r.t. | 0 | 59 |
| 2 | AuCl | — | DCE | 80–90 | 30 | 20 |
| 3 | Cu(OTf)2 | — | DCE | 80–90 | 31 | 15 |
| 4 | Cu(OTf)2 | — | DMF | 80–90 | 65 | 0 |
| 5 | CuCl2·H2O | — | DMF | 80–90 | 72 | 0 |
| 6 | CuBr2 | — | DMF | 80–90 | 83 | 0 |
| 7 | CuCl | — | DMF | 80–90 | n.d. | 30 |
| 8 | FeCl3 | — | DMF | 80–90 | n.d. | 40 |
| 9 | Cu(NO3)2·3H2O | — | DMF | 80–90 | n.d. | 46 |
| 10d | CuCl2·H2O | Phen | DMF | 80–90 | n.d. | 28 |
| 11d | CuCl2·H2O | Phen + DBU | DMF | 80–90 | n.d. | 24 |
| 12 | — | — | DMF | 80–90 | 0 | 73 |
| 13 | CuBr2 | — | DMF | 80–90 | 61e | Trace |
| 14 | CuBr2 | — | DMF | 80–90 | 83f | 0 |
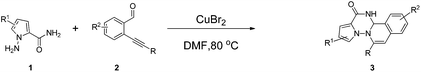
With the optimal conditions in hand, we examined the scope of the copper(II)-catalyzed annulation strategy with the results shown in Table 2. We first investigated the electron effects of substituents of 2-(phenylethynyl)benzaldehydes. When R was phenyl, it was found that the electron-withdrawing groups (–Cl, –F, –CO2CH3, –NO2) on the R afforded the target products in good to excellent yields (60–85%, Table 2, entries 1, 4, 5, 8, 12, 13, and 16). And the electron-donating groups (–CH3, –OCH3) on the R could also give moderate to excellent yields (56–92%, Table 2, entries 2, 3, 6, 9, 11, 14, and 15). The similar results were also observed when R2 was electron-withdrawing group fluoride or electron-donating group methoxyl except 2g (Table 2, entries 5, 6, 8, and 9). We proposed that the two electron-donating groups of 2g reduced the electrophilicity at the remote end of the alkyne, which thereby reduced the efficiency of the desired transformation by a 6-endo-dig cyclization approach (Table 2, entry 7). To our delight, the reaction went smoothly when R was alkyl or substituted alkyl. For example, alkyne 2j bearing a cyclopropyl group provided the target product 3j in 77% yield (Table 2, entry 10). Alkyne 2l and 2n, containing the aliphatic chain with hydroxyl group, were also suitable substrates for this reaction with excellent yields (71–94%, Table 2, entries 18, 19, 21, 22). We also investigated the electron effects of substituents on N-amino pyrrole-2-carboxyamide. The results showed that the electron-withdrawing group (–CO2CH3) gave much lower yields than that of un-substituted substrates (Table 2, entry 2 vs. 14, 3 vs. 11, 4 vs. 13, 8 vs. 16, 18 vs. 19, and 21 vs. 22) and the electron-donating group (-isopropyl) was also tolerated in this reaction (Table 2, entry 23).
| Entry | 1 | 2 | 3 | Yieldb (%) | Entry | 1 | 2 | 3 | Yieldb (%) |
|---|---|---|---|---|---|---|---|---|---|
a Reaction conditions: 1 (0.2 mmol), 2 (0.24 mmol), CuBr2 (0.02 mmol), DMF (3 ml), 80 °C, 4 h.b Isolated yield.c An inseparable mixture of unidentified products was obtained.d An inseparable mixture of 3l and its brominated product in a ratio of 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 detected by LC-MS.e The pure compound 3l was obtained in the presence of Cu(OTf)2. 17 detected by LC-MS.e The pure compound 3l was obtained in the presence of Cu(OTf)2. |
|||||||||
| 1 |  |
 |
 |
83 | 13 | 1b | 2d |  |
81 |
| 2 | 1a |  |
 |
79 | 14 | 1b | 2b |  |
92 |
| 3 | 1a |  |
 |
56 | 15 | 1b | 2i |  |
73 |
| 4 | 1a |  |
 |
75 | 16 | 1b | 2h |  |
85 |
| 5 | 1a |  |
 |
64 | 17 | 1b | 2j |  |
72 |
| 6 | 1a |  |
 |
78 | 18 | 1b |  |
 |
84 |
| 7 | 1a |  |
 |
—c | 19 | 1a | 2l |  |
71 |
| 8 | 1a |  |
 |
60 | 20 | 1b |  |
 |
64 |
| 9 | 1a |  |
 |
75 | 21 | 1b |  |
 |
94 |
| 10 | 1a |  |
 |
77 | 22 | 1a | 2n |  |
75 |
| 11 | 1a | 2c |  |
81 | 23 |  |
2a |  |
71 |
| 12 |  |
 |
 |
67d | |||||
| 40e | |||||||||
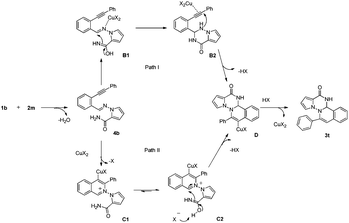
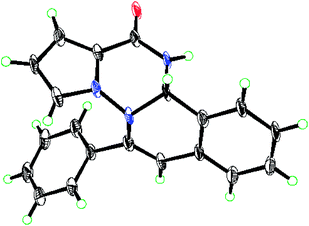
On the basis of all the above results, the mechanism hypothesis, by taking the reaction of 1b and 2m as an example, has been proposed and shown in Scheme 3. At first, Condensation between 1b and 2m would generate imine 4b. Imine 4b can be converted to the final product 3t through a cascade process proposed in two approaches. In path I, intermediate B2, formed by the attack of the nitrogen of the tautomer of amide at the imine, would carry on regiospecific hydroamination reaction, leading to the formation of D. In path II, copper-coordinated isoquinolinium cation C1, formed by nucleophilic attack of imine nitrogen atom at the alkynyl group, would be trapped by the tautomer of pyrrolo-2-amide, with the result of formation of D. Finally protonation might then occur to give compound 3t. Furthermore, the structure of compound 3t was determined by the X-ray crystallographic analysis (Fig. 2).
Conclusions
In conclusion, we have developed a Cu(II)-catalyzed coupling–cyclization reaction that allowed facile access to an impressive variety of pyrrolo[2,1-f][1,2,4]triazine-fused isoquinolines in good to excellent yields. The reaction proceeded with high 6-endo-dig regioselectivity, and the product was confirmed by X-ray crystallographic study. This method appeared to be compatible with different substituted starting materials that have different electronic properties, increasing its applicability to various functional groups. Furthermore this reaction is easy to operate and atom-economical, which will make it attractive for the construction of variety of bioactive heterocyclic compounds. Currently biological evaluation of these compounds is under investigation.Experimental section
Reagents (chemicals) were purchased from commercial sources, and used without further purification. Analytical thin layer chromatography (TLC) used was HSGF 254 (0.15–0.2 mm thickness, Yantai Jiangyou company, China). Nuclear magnetic resonance spectra were recorded on Varian Mercury-300 and/or Varian Mercury-500 spectrometers with TMS as internal standard. Chemical shift were reported in parts per million (ppm, δ) downfield from TMS. Low- and high-resolution mass spectra (LRMS and HRMS) were recorded on a Finnigan/MAT-95 spectrometer. Melting points (m.p.) were measured by Büchi 510 melting point apparatus and were uncorrected.General procedure for the synthesis of 2f, 2g and 2l
To a stirred solution of 2-bromobenzaldehyde (2 mmol) and terminal aromatic alkynes (2.4 mmol) in Et3N (10 ml) was added PdCl2(PPh3) (0.04 mmol) and CuI (0.02 mmol). The resulted mixture was heated under a nitrogen atmosphere at 50 °C for 4 h and then cooled to room temperature. After the separation of ammonium salt by filtration and removing of solvent under reduced pressure, the residue was purified by column chromatography on silica gel to afford the corresponding 2-(arylethyl)benzaldehyde 2f, 2g and 2l.General procedure for the AuCl-catalyzed reaction for synthesis of 4a and 4b
To a DCE (3 ml) solution of 1a (0.2 mmol) and 2a (0.24 mmol) in 10 ml two-neck flask was added AuCl (10 mmol%) and some molecular sieves under a nitrogen atmosphere. The mixture was stirred at room temperature for 0.5–1 hour. The white precipitate was collected by filtration, washed by water and ether, and then dried in vacuo to obtain white solid 4a.General procedure for the Cu(II)-catalyzed annulation reaction
In a 10 ml reaction tube, N-amino-1H-pyrrole-2-carboxyamide (0.2 mmol), 2-alkynylbenzaldehyde (0.24 mmol), CuBr2 (0.02 mmol) were mixed and stirred at room temperature for 5 min. Then the system was vigorously stirred at 80–90 °C for 4 h. After completion of the reaction, the mixture was poured into water, and then extracted with ethyl acetate (10 ml × 3). The combined organic layer was washed by saturated brine, and then concentrated in vacuo to afford the crude product. The pure product was obtained after purification of the residue by silica gel column chromatography (eluted with petroleum ether–ethyl acetate 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
Acknowledgements
This work was financially supported by the Chinese Academy of Sciences (‘Interdisciplinary Cooperation Team’ Program for Science and Technology Innovation), the National Natural Science Foundation of China (21072205, 81202398), and SKLDR/SIMM (SIMM1105KF-02).Notes and references
- (a) I. P. Beletskaya and V. P. Ananikov, Chem. Rev., 2011, 111, 1596 CrossRef CAS PubMed; (b) I. Nakamura and Y. Yamamoto, Chem. Rev., 2004, 104, 2127 CrossRef CAS PubMed; (c) A. V. Gulevich, A. S. Dudnik, N. Chernyak and V. Gevorgyan, Chem. Rev., 2013, 113, 3084 CrossRef CAS PubMed; (d) I. Ojima, M. Tzamarioudaki, Z. Li and R. J. Donovan, Chem. Rev., 1996, 96, 635 CrossRef CAS PubMed.
- (a) E. M. Beccalli, G. Broggini, M. Martinelli and S. Sottocornola, Chem. Rev., 2007, 107, 5318 CrossRef CAS PubMed; (b) J. Y. K. Seung hwan Cho, J. Kwak and S. Chang, Chem. Soc. Rev., 2011, 40, 5068 RSC; (c) N. Kuhl, M. N. Hopkinson, J. Wencel-Delord and F. Glorius, Angew. Chem., Int. Ed., 2012, 51, 10236 CrossRef CAS PubMed; (d) V. P. Mehta and B. Punji, RSC Adv., 2013, 3, 11957 RSC; (e) J. Bariwal and E. Van der Eycken, Chem. Soc. Rev., 2013, 42, 9283 RSC.
- (a) G. Liu, S. Abraham, L. Tran, T. D. Vickers, S. Xu, M. J. Hadd, S. Quiambao, M. W. Holladay, H. Hua, J. M. Ford Pulido, R. N. Gunawardane, M. I. Davis, S. R. Eichelberger, J. L. Apuy, D. Gitnick, M. F. Gardner, J. James, M. A. Breider, B. Belli, R. C. Armstrong and D. K. Treiber, J. Med. Chem., 2012, 55, 3250 CrossRef CAS PubMed; (b) G. R. Ott, G. J. Wells, T. V. Thieu, M. R. Quail, J. G. Lisko, E. F. Mesaros, D. E. Gingrich, A. K. Ghose, W. Wan, L. Lu, M. Cheng, M. S. Albom, T. S. Angeles, Z. Huang, L. D. Aimone, M. A. Ator, B. A. Ruggeri and B. D. Dorsey, J. Med. Chem., 2011, 54, 6328 CrossRef CAS PubMed.
- (a) C. Liu, J. Lin, S. T. Wrobleski, S. Lin, J. Hynes, H. Wu, A. J. Dyckman, T. Li, J. Wityak, K. M. Gillooly, S. Pitt, D. R. Shen, R. F. Zhang, K. W. McIntyre, L. Salter-Cid, D. J. Shuster, H. Zhang, P. H. Marathe, A. M. Doweyko, J. S. Sack, S. E. Kiefer, K. F. Kish, J. A. Newitt, M. McKinnon, J. H. Dodd, J. C. Barrish, G. L. Schieven and K. Leftheris, J. Med. Chem., 2010, 53, 6629 CrossRef CAS PubMed; (b) J. Hynes, A. J. Dyckman, S. Lin, S. T. Wrobleski, H. Wu, K. M. Gillooly, S. B. Kanner, H. Lonial, D. Loo, K. W. McIntyre, S. Pitt, D. R. Shen, D. J. Shuster, X. Yang, R. Zhang, K. Behnia, H. Zhang, P. H. Marathe, A. M. Doweyko, J. S. Tokarski, J. S. Sack, M. Pokross, S. E. Kiefer, J. A. Newitt, J. C. Barrish, J. Dodd, G. L. Schieven and K. Leftheris, J. Med. Chem., 2007, 51, 4 CrossRef PubMed.
- T. W. Wong, F. Y. Lee, S. Emanuel, C. Fairchild, J. Fargnoli, B. Fink, A. Gavai, A. Hammell, B. Henley, C. Hilt, J. T. Hunt, B. Krishnan, D. Kukral, A. Lewin, H. Malone, D. Norris, S. Oppenheimer, G. Vite and C. Yu, Clin. Cancer Res., 2011, 17, 4031 CrossRef CAS PubMed.
- A. V. Gavai, B. E. Fink, D. J. Fairfax, G. S. Martin, L. M. Rossiter, C. L. Holst, S.-H. Kim, K. J. Leavitt, H. Mastalerz, W.-C. Han, D. Norris, B. Goyal, S. Swaminathan, B. Patel, A. Mathur, D. M. Vyas, J. S. Tokarski, C. Yu, S. Oppenheimer, H. Zhang, P. Marathe, J. Fargnoli, F. Y. Lee, T. W. Wong and G. D. Vite, J. Med. Chem., 2009, 52, 6527 CrossRef CAS PubMed.
- J. E. Dinchuk, C. Cao, F. Huang, K. A. Reeves, J. Wang, F. Myers, G. H. Cantor, X. Zhou, R. M. Attar, M. Gottardis and J. M. Carboni, Endocrinology, 2010, 151, 4123 CrossRef CAS PubMed.
- (a) Y. Chen, H. Xiang, C. Tan, Y. Xie and C. Yang, Tetrahedron, 2013, 69, 2714 CrossRef CAS PubMed; (b) S. A. Patil, B. A. Otter and R. S. Klein, J. Heterocycl. Chem., 1994, 31, 781 CrossRef CAS; (c) K. S. Kim, S. Lu, L. A. Cornelius, L. J. Lombardo, R. M. Borzilleri, G. M. Schroeder, C. Sheng, G. Rovnyak, D. Crews, R. J. Schmidt, D. K. Williams, R. S. Bhide, S. C. Traeger, P. A. McDonnell, L. Mueller, S. Sheriff, J. A. Newitt, A. T. Pudzianowski, Z. Yang, R. Wild, F. Y. Lee, R. Batorsky, J. S. Ryder, M. Ortega-Nanos, H. Shen, M. Gottardis and D. L. Roussell, Bioorg. Med. Chem. Lett., 2006, 16, 3937 CrossRef CAS PubMed; (d) J. Wang, X. Wang, Y. Chen, S. Chen, G. Chen, L. Tong, L. Meng, Y. Xie, J. Ding and C. Yang, Bioorg. Med. Chem. Lett., 2012, 22, 339 CrossRef CAS PubMed; (e) T. Saito, T. Obitsu, H. Kohno, I. Sugimoto, T. Matsushita, T. Nishiyama, T. Hirota, H. Takeda, N. Matsumura, S. Ueno, A. Kishi, Y. Kagamiishi, H. Nakai and Y. Takaoka, Bioorg. Med. Chem., 2012, 20, 1122 CrossRef CAS PubMed; (f) T. Thieu, J. A. Sclafani, D. V. Levy, A. McLean, H. J. Breslin, G. R. Ott, R. P. Bakale and B. D. Dorsey, Org. Lett., 2011, 13, 4204 CrossRef CAS PubMed.
- (a) V. Sridharan, P. A. Suryavanshi and J. C. Menéndez, Chem. Rev., 2011, 111, 7157 CrossRef CAS PubMed; (b) Q. Huang, J. A. Hunter and R. C. Larock, Org. Lett., 2001, 3, 2973 CrossRef CAS PubMed; (c) D. Pla, A. Marchal, C. A. Olsen, A. Francesch, C. Cuevas, F. Albericio and M. Álvarez, J. Med. Chem., 2006, 49, 3257 CrossRef CAS PubMed; (d) E.-J. Park, E. Kiselev, M. Conda-Sheridan, M. Cushman and J. M. Pezzuto, J. Nat. Prod., 2011, 75, 378 CrossRef PubMed; (e) M. A. Rashid, K. R. Gustafson and M. R. Boyd, J. Nat. Prod., 2001, 64, 1249 CrossRef CAS PubMed; (f) C. W. Wright, S. J. Marshall, P. F. Russell, M. M. Anderson, J. D. Phillipson, G. C. Kirby, D. C. Warhurst and P. L. Schiff, J. Nat. Prod., 2000, 63, 1638 CrossRef CAS PubMed; (g) H. Sorek, A. Rudi, I. Goldberg, M. Aknin and Y. Kashman, J. Nat. Prod., 2009, 72, 784 CrossRef CAS PubMed; (h) D. S. Bhakuni and S. Gupta, J. Nat. Prod., 1982, 45, 407 CrossRef CAS.
- (a) X. Yao and C.-J. Li, Org. Lett., 2006, 8, 1953 CrossRef CAS PubMed; (b) M. Chrzanowska and M. D. Rozwadowska, Chem. Rev., 2004, 104, 3341 CrossRef CAS PubMed.
- H. Gao and J. Zhang, Adv. Synth. Catal., 2009, 351, 85 CrossRef CAS.
- N. T. Patil, A. K. Mutyala, P. G. V. V. Lakshmi, P. V. K. Raju and B. Sridhar, Eur. J. Org. Chem., 2010, 1999 CrossRef CAS.
- P. M. k. K. Siva Kumar, M. Appi Reddy and Md. Ferozudlin, Chem. Commun., 2011, 46, 10263 RSC.
- (a) H. Xiang, Y. Chen, Q. He, Y. Xie and C. Yang, RSC Adv., 2013, 3, 5807 RSC; (b) M. Wang, C. Tan, Q. He, Y. Xie and C. Yang, Org. Biomol. Chem., 2013, 11, 2574 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 909484. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3ra47324d |
| This journal is © The Royal Society of Chemistry 2014 |