Rapid and selective detection of Cys in living neuronal cells utilizing a novel fluorescein with chloropropionate–ester functionalities†
Abstract
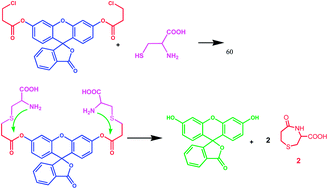
A chloropropionate-caged fluorescein probe allows for prompt detection of cysteine over other biothiols, e.g., homocysteine with a limit of detection of 12.8 μM.

 Please wait while we load your content...
Please wait while we load your content...