Time-dependent control of phase and morphology transformation of porous ZnO hollow microspheres by a facile one-step solution route
Linxia Fangab,
Baoliang Zhanga,
Wei Lia,
Xiangjie Lia,
Tiejun Xina and
Qiuyu Zhang*a
aDepartment of Applied Chemistry, School of Science, Northwestern Polytechnical University, 710072 Xi'an, China. E-mail: qyzhang1803@gmail.com
bCollege of Chemistry and Chemical Engineering, Xinyang Normal University, 464000 Xinyang, China
First published on 7th January 2014
Abstract
Porous ZnO hollow microspheres with a ZnO crystalline phase of PDF # 21-1486 have been prepared by a simple one-step solution route using the trisodium citrate as a shape-directing agent. FESEM, FT-IR, TEM and XRD were used to characterize the samples. Series of experiments showed the reaction time has a prominent impact on the phase and morphology transformation. At a point of the controlling growth time, porous ZnO hollow microspheres with average diameter of 2–3 μm and hole-opening diameter of ∼0.5 μm were obtained which exhibited a high surface area (117.36 m2 g−1) and a large pore volume (0.50 cm3 g−1). On the basis of experiment results, a possible growth mechanism of as-synthesized porous ZnO hollow microspheres was concluded to be a two-step process: the zinc citrate complexion was preferentially formed, and then the dissolution of zinc citrate complexion and the formation of ZnO crystal occurred simultaneously, which associated with the special role of the citrate anion on oriented growth.
1. Introduction
Hollow micro- and nano-structures have attracted great interest because of their promising applications in a wide range of areas.1–3 Numerous studies have been focused on the facile synthesis of hollow structures with different complex interiors,4–6 in view of their remarkable architectures and tunable physical and chemical properties, which are attractive for many applications.7–17ZnO is a versatile semiconductor material with a direct wide band gap (3.37 eV) and large exciton binding energy (60 meV) at room temperature and has been extensively studied as light-emitting diodes,18,19 solar cells,20–24 lasers,25–27 and sensors28–30 due to its unique physics and chemistry properties. In particular, porous or hollow-structured ZnO materials have drawn special interest because of their advantageous features including low density, high surface area, nanostructure wall, good surface permeability, hollow nature and distinct optical attributes. To obtain ideal properties for some specific applications, different synthetic strategies have been extensively studied and various ZnO nanostructures have been prepared.31–41 However, the controllable fabrication of ZnO hollow structures still remains very challenging because the synthesis methods always suffer from high temperature, expensive substrates, high cost, need of sophisticated equipments and rigorous experimental conditions.
In this article, we reported the controllable synthesis of well-defined porous ZnO hollow microspheres with an open gap and a high surface area, employing trisodium citrate as structure-directing agent, through a facile one-step solution route. It was worth pointing out that the diffraction pattern of as-synthesized porous ZnO hollow sphere was characteristic of a ZnO phase of PDF # 21-1486. The experiments verified that the morphology and phase of the sample can be controlled simply by adjusting the reaction time. Several groups have reported the synthesis of porous ZnO microspheres in the presence of trisodium citrate,42–47 but the phase transformation of this process has hardly been investigated. In this work, we gain a different growth mechanism on the basis of the experimental results. The research results may provide reference for the design and synthesis of porous or hollow nano/micro-structure materials.
2. Experimental techniques
2.1. Materials
Zinc acetate dehydrate, hexamine (HMTA), absolute ethanol and sodium citrate were obtained from China National Pharmaceutical Industry Corporation Ltd. All chemicals used in this work were of analytical grade and were used without further purification.2.2. Synthesis of ZnO crystals
In a typical synthesis, an equimolar ratio of zinc acetate dehydrate (25 mM) and HMTA (25 mM) was dissolved into 50 mL deionized water with subsequent addition of trisodium citrate (5 mM), followed by stirring at room temperature for 20 min. The final mixture was transferred to a 100 mL Teflon-stainless beaker for hydrolysis reaction at 90 °C in an oven. After cooling to room temperature naturally, the resulting white precipitate was collected by centrifugation, washing with deionized water and absolute ethanol several times, and dried at 60 °C for 24 h.2.3. Characterization of ZnO crystals
Scanning electron microscopy (SEM) observation was performed on a JEOL JSM-6480A scanning electron microscope. The crystalline structure of the samples was studied by Xray diffraction (XRD) spectroscopy, using a Bruker Inc. (Germany) AXS D8 ADVANCE diffractometer (Cu Kα radiation). Transmission electron microscopy (TEM) observation was performed on a FEI Tecnai G2S-Twin transmission electron microscope (TEM) with an accelerating voltage of 200 kV. The Brunauer–Emmett–Teller (BET) surface area of the ZnO hollow sphere sample was tested using Quanta chrome NOVA 2000e sorption analyzer. Fourier transform infrared (FT-IR) spectra was collected on a Brucker TENSOR 27 spectrometer.3. Results and discussion
3.1. Morphologies and structures of the ZnO hollow microsphere
Fig. 1 and 2 show typical FESEM, TEM and HRTEM images of the ZnO hollow spheres formed at the reaction time of 10 h. The low magnification image (Fig. 1a) clearly shows that the sample consists of relatively uniform, well-dispersed microspheres with average diameter of 2–3 μm (Fig. 1c) and hole-opening diameter of ∼0.5 μm. The magnified SEM image (Fig. 1b) shows its porous surface. Especially, the open pores on the spheres obviously certify the sphere is hollow, which can also be verified by the following TEM observation. The TEM image (Fig. 2a) displays the porous spherical-shaped microstructures with a hollow interior. The HRTEM image (Fig. 2b) taken from the edge of a single ZnO microsphere further confirms the nanostructure wall consisting of thin nanosheets. | ||
| Fig. 1 (a) Low, (b) high magnification FESEM images and (c) diameter distribution of hollow ZnO microspheres. | ||
Fig. 3 shows (a) the pore volume and (b) the pore size distribution of the as-synthesized porous ZnO hollow microspheres which were analyzed from N2 adsorption–desorption isotherms based on the BJH (Barrett–Joyner–Halenda) model. The ZnO hollow microspheres exhibit high surface area, large pore volume, and narrow pore size distribution. At high P/P0 between 0.4 and 1.0, the samples exhibit a type H1 hysteresis loop (IUPAC classification) indicating the porous nature of ZnO hollow microspheres. The BET (Brunauer–Emmett–Teller) surface area and the pore volume are found to be 117.36 m2 g−1 and 0.50 cm3 g−1 respectively. The pore size distribution curve of the ZnO hollow microspheres suggests an average pore diameter of 18.2 nm.
 | ||
| Fig. 3 (a) Nitrogen adsorption–desorption isotherm and (b) BJH pore size distribution curve of the ZnO hollow microspheres. | ||
3.2. Effect of reaction time on phase and morphology
To explore the overall growth process of the hollow ZnO microspheres, experiments have been performed by adjusting reaction time and keeping other reaction parameters constant. Fig. 4 shows the low (left) and high (right) magnification FESEM images of the samples synthesized for 2 h (a), 4 h (b), 6 h (c) and 7 h (d), respectively. In the preliminary stage of 2 h, it is seen that the sample mainly consists of microspheres accompanied with few cloudy materials. There is a thin layer of loose materials on the surface of the sphere and some tiny flakes falling off from the surface. As the reaction time increases to 4 h, the low magnification FESEM images show an obvious change that there are some pill-like materials randomly adhering on the surface (b1).The high magnification FESEM images of ZnO obtained at different reaction times: 2 h (a), 4 h (b), 6 h (c) and 7 h (d), show the exterior pill-like material is fleecy and the interior sphere is smooth (b2). With further increase of the reaction time to 6 h, the surfaces of the spheres are completely coated by the fleecy materials as a shell (c1). The high magnification FESEM image shows the bigger piece of fleecy materials piling up on the surface of the microsphere which make it look like pineapple peel, which is coarse and fleecy (c2). Further increase of the reaction time to 7 h, a striking change is observed that the microsphere is hole-opening. It can found the hollow interior through the hole (d1 and d2). When the reaction time reaches 10 h, the diameter of the hole increases and the diameter of microspheres decreases, coupling with the shell becoming porous (Fig. 1). As a result, the well-dispersed, uniform hollow microsphere with nanosheets stacking shell is obtained. The diameter of microsphere is 2–3 μm and the hole-opening diameter is about 0.5 μm. When the reaction time further increases to 15 h, the adhesion contact between the spheres happens, which leads to the deformation of the microspheres, even forming irregular shape (Fig. 5a). When the reaction time extends to 20 h, the most microspheres are distorted and even collapsed (Fig. 5b). So, 10 h was conceived as the optimum growth time for the synthesis of porous ZnO hollow microspheres.
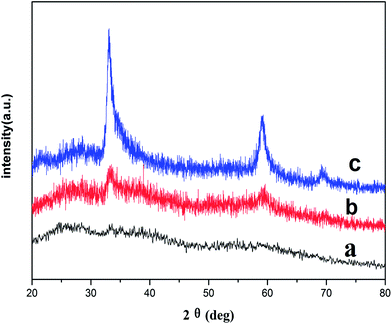
In order to further reveal the formation process of the porous ZnO hollow microsphere, the XRD patterns of the samples obtained at reaction times of 2 h, 4 h and 10 h were measured (Fig. 6). When reaction time is 2 h, no patterns or peaks are observed and the obtained sample is amorphous (Fig. 6a). When reaction time is 4 h, there are weak diffraction peaks in the XRD pattern which are agreed with that of a ZnO phases (JCPDS 21-1486) (Fig. 6b), which indicates that the sample begin to crystal. That is to say, 4 h is the point of time of the phase transformation for ZnO from amorphous phase to crystal phase. Interestingly, as shown in Fig. 6c, the diffraction peaks in XRD pattern locating at 33.6°, 58.7° and 69.5°are consistent with the literature data of JCPDS 21-148648 and not the common wurtzite ZnO (JCPDS no. 36-1451). This crystal structure also was detected by a few researchers. Rykl et al.49 first obtained this ZnO phase under the condition of high temperature (450 °C) and high pressure (600 bar). Yue Zhao et al.50 reported this ZnO phase in heavy N-dopped (10%) ZnO film. It is noted that the JCPDS file only gives Bragg angle and relative intensities, and no information is provided about the crystal structure. Combining with the SEM image of the sample at 4 h (Fig. 4, b2), we can conclude the pill-like fleecy material coating outside the smooth spheres is crystal. The XRD pattern of the sample at 10 h (Fig. 6c) shows the sharp and strong diffraction peaks indexed to ZnO phases (JCPDS 21-1486), which further illustrates that the porous nanostructure shell is crystal and assigned to the ZnO phase (JCPDS 21-1486).
To obtain more information about the samples, FT-IR spectra were also measured. Fig. 7 shows the FT-IR spectra of the samples obtained at different reaction times (2 h, 4 h and 10 h). As shown in (a) and (b), the strong absorption peaks at 1360 and 1600 cm−1 are assigned as the symmetric and asymmetric stretching bands of COO− of the coordinated citrates,51 which confirms the existence of citrate. Because the sample synthesized for 2 h is amorphous, we can speculate citrate anion exists in the forms of zinc citrate complexion and this zinc citrate complexion is the building block of the amorphous sample. The weak peak at ca. 437 cm−1 derived from Zn–O is presented in Fig. 7b, which suggests the formation of ZnO when reaction time is 4 h. In the case of 10 h reaction time, there is no other peak but the obvious ZnO peak at ca. 437 cm−1, as shown in Fig. 7c, which strongly implies that the sample is ZnO. This result is consistent with the XRD.
3.3. Effect of annealing on the phase and morphology
Fig. 8 shows the FESEM images (a) and XRD pattern (b) of the as-prepared hollow ZnO microsphere after annealing under 500 °C for 30 min. The high magnification FESEM image shows the hollow koosh ball micostructure with a hole-opening after annealing. The diffraction peaks in the X-ray diffraction pattern (b) of the heat-treated sample are well matched with wurtzite ZnO (JCPDS no. 36-1451), which indicates the ZnO crystal phase (PDF # 21-1486) was completely converted to wurtzite ZnO (JCPDS no. 36-1451) after annealing. | ||
| Fig. 8 (a) FESEM images, (b) XRD pattern of hollow ZnO microsphere after annealing under 500 °C for 30 min, respectively. | ||
3.4. Growth mechanism of the ZnO nanocrystals
Nirmalya T. et al.43 employed a two-step route to prepare hollow ZnO microspheres. They thought initial formation of Zn(OH)2 microspheres, then the formation of the ZnO surface of microspheres and subsequent dissolution of internal Zn(OH)2 were the growth mechanism of hollow ZnO microspheres. Lan Ge et al.44 also obtained hollow ZnO microspheres with the assistant of trisodium citrate and proposed the growth mechanism that at the beginning, numerous growth units of ZnO were generated and gathered to the bubble surface of formaldehyde and excess ammonia, produced by thermal decomposition of methenamine in solution, and subsequently, these units tend towards relatively faster equatorial growth, and form spherical building blocks by the assistance of trisodium citrate. However, series of time-dependent experiments carried out in this paper indicated a different viewpoint. More possible growing mechanism proposed by us can be illustrated in Fig. 9. During the formation of ZnO hollow microspheres, some chemical reactions might occur as listed in (1) to (5).51,52| C6H12N4 + 6H2O → 6HCHO + 4NH3 | (1) |
| NH3 + H2O → NH4 + OH− | (2) |
| Zn2+ + C6H5O73− → Zn2+–citrate | (3) |
| Zn2+–citrate + 4OH− → Zn(OH)42− | (4) |
| Zn(OH)42− → ZnO + H2O | (5) |
From XRD (Fig. 7) and FT-IR (Fig. 8), the basic building unit of the solid sphere was zinc citrate complexion and not ZnO at an early stage (before 2 h). In principle, a nucleation in a solution occurs when a surrounding concentration exceeds a critical concentration. The concentration of OH− was not enough to reach saturation at the preliminary stage because the OH− in the solution comes from the slowly hydrolysis of HTMA. So there is no ZnO forming and only lots of Zn2+ and citrate anion in the solution, and the binding of Zn2+ and citrate firstly produced amorphous zinc citrate complexion.53,54 The surface areas of the amorphous structures tend to minimize in the reaction solution. Thus, the synthesized structures are spherical in shape, with a minimized surface area for the given volume. It has been reported that the metal–citrate complex is relatively stable in an acid condition but easily decomposes in an alkaline condition.55 With the increase of the reaction time, the concentration of OH− increased. The low concentration of OH− in the solution can promote the dissolution of the surface of the amorphous zinc citrate microspheres by reacting with Zn2+, which makes the superficial layers loose and even some tiny flakes fall down, generating the growth unit of Zn(OH)42− and releasing citrate anions. The dissolution also occurs followed with the falling tiny flakes. As the increasing reaction time, the concentration of OH− further increases, nucleation and subsequent growth of ZnO crystals occurs on surface of the amorphous microsphere and the surface of the tiny flakes when the concentration of (Zn(OH)42−) reaches saturation. Since citrate anion is easily absorbed on the metal ion, the ZnO nanoparticles on the microspheres or the tiny flakes develop gradually to nanosheets through hydrogen-bonding interactions between the citrate anions,31 and then join to each other to form network, which adheres on the surface of the microspheres and makes the surface fleecy and coarse. With the further dissolution of the amorphous zinc citrate, more ZnO networks are formed, and the sphere is found completely coated with fleecy material to form a pineapple peel-look surface. The OH− not only reacts with zinc citrate in the shell but also penetrates through the outer layer of the microsphere to etch internal sphere. Such, the internal sphere becomes smaller and finally disappear, and the pore size of the networks increases coupling with the nanosheets thickness decreased. As a result, the hollow ZnO microsphere with nanosheets stacking shell is formed. The appearance of the hole on the microsphere is contributed to the structural inhomogeneity of the microsphere produces the unbalanced stress which leads to the formation of a gap at the weakest point. The formation of the ZnO phase of PDF # 21-1486 is due to the citrate anion absorbing on the ZnO nucleus distorted the lattice which changes packing patterns of Zn and O atomics in the crystal. These results suggest that citrate anion plays an important role in the formation of the porous ZnO hollow microsphere and the ZnO phase of PDF # 21-1486.
Based on the above analysis, a two-step process could be an underlying growth mechanism for as-prepared hollow ZnO microsphere: the zinc citrate complexion was preferentially formed, and then the dissolution of zinc citrate complexion and the formation of ZnO crystal occurred simultaneously, which associated with the special role of the citrate anion on oriented growth. This growth mechanism is valuable for a facial one-step solution route to prepare porous ZnO hollow microsphere only by controlling reaction time.
4. Conclusions
In summary, a simple, cost-effective, one-step solution method was employed to prepare porous ZnO hollow microsphere in the presence of trisodium citrate. The as-synthesized ZnO hollow microspheres were assigned to a ZnO phase (PDF # 21-1486). It showed a high surface area (117.36 m2 g−1) and a large pore volume (0.50 cm3 g−1). Investigations showed that the reaction time affected the formation of the phase and morphology of ZnO microsphere. With the reaction time increasing from 2 h to 20 h, the samples transformed from amorphous zinc citrate complexion microspheres to porous ZnO hollow microspheres. The progressive dissolution of zinc citrate complexion and formation of ZnO crystal were associated with oriented growth and connection by the citrate anion could be an underlying mechanism for understanding the evolution of the phase and morphology of the samples.Acknowledgements
The authors are grateful for the financial support provided by National High Technology Research and Development Program of China (no. 2012AA02A404), National Natural Science Foundation of China (no. 51173146), basic research fund of Northwestern polytechnical university (JC20120248) and the Natural Science Foundation of Henan Province (nos 132300410406).References
- A. Pan, H. B. Wu, L. Yu and X. W. David Lou, Angew. Chem., 2013, 125, 2282 CrossRef.
- X. W. Lou, L. A. Archer and Z. Yang, Adv. Mater., 2008, 20, 1853 CrossRef CAS.
- F. Bai, Z. Sun, H. Wu, R. E. Haddad, X. Xiao and H. Fan, Nano Lett., 2011, 11, 3759 CrossRef CAS PubMed.
- J. W. Hong, S. W. Kang, B.-S. Choi, D. Kim, S. B. Lee and S. W. Han, ACS Nano, 2012, 6, 2410 CrossRef CAS PubMed.
- W. Zhang, J. Yang and X. Lu, ACS Nano, 2012, 6, 7397 CrossRef CAS PubMed.
- M. F. Lin, V. Kumar Thakur, E. J. Tan and P. S. Lee, J. Mater. Chem., 2011, 21, 16500 RSC.
- Z. Dong, X. Lai, J. E. Halpert, N. Yang, L. Yi, J. Zhai, D. Wang, Z. Tang and L. Jiang, Adv. Mater., 2012, 24, 1046 CrossRef CAS PubMed.
- M. Ye, X. Xin, C. Lin and Z. Lin, Nano Lett., 2011, 11, 3214 CrossRef CAS PubMed.
- X. Fang, X. Zhao, W. Fang, C. Chen and N. Zheng, Nanoscale, 2013, 5, 2205 RSC.
- C. Qi, Y. Zhu, B. Lu, X. Zhao, J. Zhao, F. Chen and J. Wu, Chem. – Eur. J., 2013, 19, 5332 CrossRef CAS PubMed.
- S. D. Shinde, G. E. Patil, D. D. Kajale, V. B. Gaikwad and G. H. Jain, J. Alloys Compd., 2012, 518, 109 CrossRef PubMed.
- X. H. Huang, X. H. Xia, Y. F. Yuan and F. Zhou, Electrochim. Acta, 2011, 56, 4960 CrossRef CAS PubMed.
- Z. Wu, L. Qin and Q. Pan, J. Alloys Compd., 2011, 509, 9207 CrossRef CAS PubMed.
- M. Ahmad, S. Yingying, A. Nisar, H. Sun, W. Shen, M. Wei and J. Zhu, J. Mater. Chem., 2011, 21, 7723 RSC.
- Z. Wang, L. Zhou and X. W. David Lou, Adv. Mater., 2012, 24, 1903 CrossRef CAS.
- G. Tian, Y. Chen, W. Zhou, K. Pan, Y. Dong, C. Tian and H. Fu, J. Mater. Chem., 2011, 21, 887 RSC.
- W. Tu, Y. Zhou, Q. Liu, Z. Tian, J. Gao, X. Chen, H. Zhang, J. Liu and Z. Zou, Adv. Funct. Mater., 2012, 22, 1215 CrossRef CAS.
- D. I. Son, B. W. Kwon, D. H. Park, W.-S. Seo, Y. Yi, B. Angadi, C.-L. Lee and W. K. Choi, Nat. Nanotechnol., 2012, 7, 465 CrossRef CAS PubMed.
- Q. Qiao, B. H. Li, C. X. Shan, J. S. Liu, J. Yu, X. H. Xie, Z. Z. Zhang, T. B. Ji, Y. Jia and D. Z. Shen, Mater. Lett., 2012, 74, 104 CrossRef CAS PubMed.
- J. Jean, S. Chang, P. R. Brown, J. J. Cheng, P. H. Rekemeyer, M. G. Bawendi, S. Gradecak and V. Bulovic, Adv. Mater., 2013, 25, 2790 CrossRef CAS PubMed.
- Z. Li, Y. Zhou, G. Xue, T. Yu, J. Liu and Z. Zou, J. Mater. Chem., 2012, 22, 14341 RSC.
- Y. Shi, C. Zhu, L. Wang, C. Zhao, W. Li, K. K. Fung, T. Ma, A. Hagfeldt and N. Wang, Chem. Mater., 2013, 25, 1000 CrossRef CAS.
- A. Kim, Y. Won, K. Woo, C.-H. Kim and J. Moon, ACS Nano, 2013, 7, 1081 CrossRef CAS PubMed.
- H. Park, S. Chang, J. Jean, J. J. Cheng, P. T. Araujo, M. Wang, M. G. Bawendi, M. S. Dresselhaus, V. Bulović, J. Kong and S. Gradečak, Nano Lett., 2013, 13, 233 CrossRef CAS PubMed.
- H. Zhu, C.-X. Shan, B. Yao, B.-H. Li, Ji.-Y. Zhang, Z.-Z. Zhang, D.-X. Zhao, D.-Z. Shen, X.-W. Fan, Y.-M. Lu and Z.-K. Tang, Adv. Mater., 2009, 16, 1613 CrossRef.
- X.-Y. Liu, C.-X. Shan, S.-P. Wang, Z.-Z. Zhang and D.-Z. Shen, Nanoscale, 2012, 4, 2843 RSC.
- H. Zhu, C.-X. Shan, J.-Y. Zhang, Z.-Z. Zhang, B.-H. Li, D.-X. Zhao1, B. Yao, D.-Z. Shen, X.-W. Fan, Z.-K. Tang, X. Hou and K.-L. Choy, Adv. Mater., 2010, 16, 1877 CrossRef PubMed.
- X. B. Zhao, G. M. Ashley, L. Garcia-Gancedo, H. Jin, J. K. Luo, A. J. Flewitt and J. R. Lu, Sens. Actuators, B, 2012, 163, 242 CrossRef CAS PubMed.
- S. Chawla and C. S. Pundir, Anal. Biochem., 2012, 430, 156 CrossRef CAS PubMed.
- L. K. vanVugt, S. Rühle and D. Vanmaekelbergh, Nano Lett., 2006, 6, 2707 CrossRef CAS PubMed.
- T. J. Athauda and R. R. Ozer, Cryst. Growth Des., 2013, 13, 2680 CAS.
- J. Ho Shin, J. Y. Song, Y. H. Kim, S. D. Kim and H. M. Park, Mater. Lett., 2012, 66, 106 CrossRef PubMed.
- Y.-M. Chang, J.-M. Huang, C.-M. Lin, H.-Y. Lee, S.-Y. Chen and J.-Y. Juang, J. Phys. Chem. C, 2012, 116, 8332 CAS.
- C.-Y. Kuo, R.-M. Ko, Y.-C. Tu, Y.-R. Lin, T.-H. Lin and S.-J. Wang, Cryst. Growth Des., 2012, 12, 3849 CAS.
- L. Song, S. Zhang, X. Wu and Q. Wei, Ind. Eng. Chem. Res., 2012, 51, 4922 CrossRef CAS.
- W. Feng, A. S. Wan and E. Garfunkel, J. Phys. Chem. C, 2013, 117, 9852 CAS.
- D. J. Gargas, H. Gao, H. Wang and P. Yang, Nano Lett., 2011, 11, 3792 CrossRef CAS PubMed.
- T. Gordon, M. Kopel, J. Grinblat, E. Banin and S. Margel, J. Mater. Chem., 2012, 22, 3614 RSC.
- X. Gao, H. Zhao, J. Wang, X. Su and F. Xiao, Ceram. Int., 2013, 4, 1–4 Search PubMed.
- J. Zhao, J. Yin and M. Yang, J. Alloys Compd., 2013, 579, 45–49 CrossRef CAS PubMed.
- S. Kumar, T. Surendar, D. Das, B. Kumar and V. Shanker, Mater. Lett., 2013, 101, 33 CrossRef CAS PubMed.
- Q. Xie, J. Li, Q. Tian and R. Shia, J. Mater. Chem., 2012, 22, 13541 RSC.
- N. Tripathy, R. Ahmad, H.-S. Jeong and Y.-B. Hahn, Inorg. Chem., 2012, 51, 1104 CrossRef CAS PubMed.
- L. Ge, X. Jing, J. Wang, J. Wang, S. Jamil, Q. Liu, F. Liu and M. Zhang, J. Mater. Chem., 2011, 21, 10750 RSC.
- B. Jin and D. Wang, J. Lumin., 2012, 132, 1879 CrossRef CAS PubMed.
- F. Meng, J. Yin, Y. Duan, Z. Yuan and L. Bie, Sens. Actuators, B, 2011, 156, 703–708 CrossRef CAS PubMed.
- W. Guo, T. Liu, H. Zhang, R. Sun, Y. Chen, W. Zeng and Z. Wang, Sens. Actuators, B, 2012, 166, 492 CrossRef PubMed.
- JCPDS file no. 21–1486.
- D. Rykl and J. Bauer, Kristall und Technik, 1968, 3, 375 CrossRef CAS.
- Y. Zhao, Z. Li, Z. Lv, X. Liang, J. Min, L. Wang and Y. Shi, Mater. Res. Bull., 2010, 45, 1046 CrossRef CAS PubMed.
- Z. R. Tian, J. A. Voigt, J. Liu, B. McKenzie, M. J. McDermott, M. A. Rodriguez, H. Konishi and H. Xu, Nat. Mater., 2003, 2, 821–825 CrossRef CAS PubMed.
- B. Cao, W. Cai, G. Duan, Y. Li, Q. Zhao and D. Yu, Nanotechnology, 2005, 16, 2567 CrossRef CAS.
- S. Cho, J.-W. Jang, A. Jung, S.-H. Lee, J. Lee, J. S. Lee and K.-H. Lee, Langmuir, 2011, 27, 371 CrossRef CAS PubMed.
- L. Jia, W. Cai, H. Wang and H. Zeng, Cryst. Growth Des., 2008, 8, 4369 Search PubMed.
- T. Trindade, J. D. Pedrosa de Jesus and P. O'Brien, J. Mater. Chem., 1994, 4, 1611 RSC.
| This journal is © The Royal Society of Chemistry 2014 |