Investigation on lanthanide-dependent z value of JEM-phase Sialon
Abstract
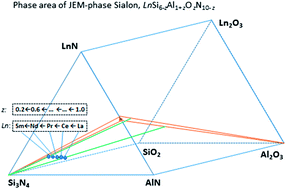
JEM-phase Sialon (LnSi6−zAl1+zOzN10−z) was revealed to have limited z values. To keep the structure stability, substitution of the Si–N bond by the Al–O bond is very restricted and lanthanide-dependent (La → Sm). The z values of La-, Nd- and Sm-doped JEM-phase Sialons are ∼1.0, ∼0.6 and 0.4–0.2, respectively.

 Please wait while we load your content...
Please wait while we load your content...