Kinetic model of carbon nanotube production from carbon dioxide in a floating catalytic chemical vapour deposition reactor
Geoffrey S. Simatea,
Kapil Moothiab,
M. Meyyappanc,
Sunny E. Iyuke*ab,
Sehliselo Ndlovua,
Rosemary Falcona and
Mike Heydenrychd
aSchool of Chemical and Metallurgical Engineering, University of the Witwatersrand, P/Bag 3, Wits 2050, Johannesburg, South Africa. E-mail: sunny.iyuke@wits.ac.za
bDST/NRF Centre of Excellence in Strong Materials, P/Bag 3, Wits 2050, Johannesburg, South Africa
cCenter for Nanotechnology, NASA Ames Research Center, Moffett Field, CA 94035, USA
dDepartment of Chemical Engineering, University of Pretoria, P/Bag X20, Hatfield 0028, South Africa
First published on 27th January 2014
Abstract
The production of carbon nanostructures, including carbon nanotubes (CNTs), by chemical vapour deposition (CVD) occurs by thermally induced decomposition of carbon-containing precursors. The decomposition of the feedstock leading to intermediate reaction products is an important step, but rarely incorporated in rate equations, since it is generally assumed that carbon diffusion through or over the catalyst nanoparticles is the rate-limiting step in the production of CNTs. Furthermore, there is no kinetic model to date for the production of CNTs from carbon dioxide. These aspects are addressed in this study with the aid of a series of experiments conducted in a floating catalytic CVD reactor in which the effects of reactor temperature, concentration and flow rate of CO2 were investigated. A simple rate equation for the reductive adsorption of CO2 onto the catalyst surface followed by carbon diffusion leading to the production of CNTs is proposed as follows: d[CNT]/dt = K[CO2], where K is proportional to the diffusion coefficient of carbon. The derived kinetic model is used to calculate the amount of CNTs for a given concentration of CO2, and the experimentally measured data fits the simple rate equation very well at low carbon dioxide concentration.
Introduction
Carbon dioxide (CO2) is the most prevalent greenhouse gas that traps heat and raises the global temperature. Interest in its use as a raw material in the synthetic chemical industry has increased over the past few decades1–4 including the possibility of using CO2 in the production of carbon nanotubes (CNTs).5 Due to their interesting electronic properties and exceptional mechanical properties, CNTs have received much attention for application in electronics, high strength composites, chemical and biosensors, field emission devices, filters and membranes, catalyst support, water purification and numerous others.6 Despite the large potential of using CNTs in various applications, the major problem is low production rate. Besides the arc discharge process, chemical vapor deposition (CVD) has been a popular technique for depositing CNTs on substrates as well as for bulk production.6 The floating catalyst CVD (FC-CVD) method shows much promise for continuous, large-scale synthesis of high purity CNTs.7 A modified version of this approach using swirled flow, known as swirled floating catalytic chemical vapour deposition (SFCCVD), has emerged recently8 and this process has been successfully applied for the growth of multi-walled CNTs (MWCNTs) and single-walled CNTs (SWCNTs).9,10 The SFCCVD method has been found to be more successful both in terms of operational practicality and CNT yield than the microwave or the fixed-bed catalytic CVD modes.10–13 In the pursuit of large scale and continuous production of CNTs, kinetic studies of their formation have continued to be a subject of interest. However, unlike CNTs produced from other carbon sources,14–17 there is a dearth of literature on the kinetic modelling of CNTs produced from CO2. The present work is devoted to the development of a simple kinetic model for the production of CNTs using CO2.Materials and methods
The CNTs were produced by a method similar to that of Xu and Huang18 using a 10% iron catalyst on calcium carbonate support. The choice of calcium carbonate was based on the good quality, high yield and high purity materials obtained from its use as a support.19 The catalyst was prepared by a modified wet impregnation technique20 by mixing a predetermined amount of iron nitrate and citric acid in approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio in deionized water. Ammonia solution was added in drops until a neutral pH was reached. A reddish brown solution with no precipitate was obtained after a period of six hours. A calculated amount of calcium carbonate was then stirred into this solution to form a dry slurry, which was then left to stand overnight. The resulting powder was calcined at 500 °C between 2 and 12 h in a muffle furnace to decompose the nitrates from the catalyst.21
1 molar ratio in deionized water. Ammonia solution was added in drops until a neutral pH was reached. A reddish brown solution with no precipitate was obtained after a period of six hours. A calculated amount of calcium carbonate was then stirred into this solution to form a dry slurry, which was then left to stand overnight. The resulting powder was calcined at 500 °C between 2 and 12 h in a muffle furnace to decompose the nitrates from the catalyst.21
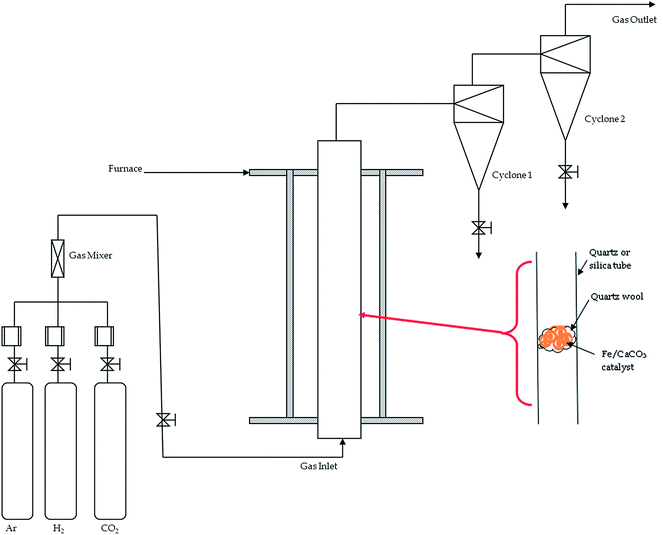
Fig. 1 shows a SFCCVD reactor used to synthesize CNTs, which consists of a vertical quartz or silica plug-flow reactor inside a furnace. The upper end of the reactor is connected to a condenser that leads to two delivery cyclones where the CNTs are collected. Feed materials including carrier gases are uniformly mixed with the aid of a swirled coiled mixer to give optimum interaction. The flow of gases into the SFCCVD reactor is aided by a system of valves and rotameters.22
Approximately 10 g of the catalyst was placed on quartz wool within a vertical silica tube (30 mm inner diameter) that was later heated by an electric furnace (Fig. 1). Once the experiment was set-up, the reactor was purged with argon to remove oxygen that could oxidise the CNTs.23 Ultra high purity (UHP) grade gases as supplied by AFROX Ltd (South Africa) were used in this study. The feedstock consisted of a mixture of CO2 (99.99% minimum purity) and hydrogen (H2) (99.99% minimum purity). Growth experiments were conducted at temperatures ranging from 740–850 °C.
Transmission electron microscopy (TEM, JEOL JEM-4010) was used to characterize the surface morphology of the CNTs. The samples for the TEM observations were prepared by ultrasonically dispersing them in 5 ml methanol (for 10 minutes) and then, depositing a drop of the solution onto a lacey carbon film on a copper grid. The degree of crystallinity and type of CNTs were evaluated by Raman spectroscopy using the standard 514.5 nm line of an argon ion laser.
Kinetic model formulation
Several models have been suggested for the growth of CNTs using catalysts, for example, the scooter model,24,25 metal-particle model,26 fullerene-cap model,27 etc. However, the vapour–liquid–solid (VLS) growth model is the most popular to explain the growth of CNTs using catalysts.28 In this model, the liquid catalyst particle plays the following roles: (i) as a catalyst for the decomposition or reduction of the carbon feedstock (e.g., CO, CO2, CH4 or alcohols), (ii) as a solvent for the carbon atoms that are released from the feedstock, and (iii) as a soft template for the nucleation and growth of the CNTs. According to the VLS growth model, the carbon feedstock is initially in the vapour phase before dissolving into the metal catalyst to form a liquid metal–carbide particle. When this particle is carbon-saturated, the solid phase CNTs begin to grow.29 It has been generally assumed that carbon diffusion through the catalyst bulk or over the catalyst nanoparticle is the only rate limiting step in the CNT growth process.30 However, CNT growth rate in CVD can be limited by several other steps such as diffusion and mass transfer in the gas phase, surface reactions on the catalyst, etc., as shown schematically30,31 in Fig. 2. Furthermore, the growth rate may also be dependent on many other factors including the gas phase decomposition reactions. Therefore, in order to understand the chemical mechanism that takes place during CNT growth from CO2, the thermal decomposition of CO2 should also be analysed and fully understood. The understanding of the thermal and kinetic aspects of gas phase decomposition reactions is critical in many fundamental and applied fields, for example, environmental sciences, combustion and explosions, catalysis, and planetary sciences.32 However, gas phase decomposition can be extremely complex due to a large number of variables, a variety of possible intermediates, and an overlap in thermal decomposition traces. Nevertheless, calculations intended to provide an interpretation of the experiments are often of little help if they ignore the kinetics of the decomposition process.32 Therefore, decomposition or reduction of CO2 into the required chemical species is considered to be an important step here for incorporation into the rate equation for the production of CNTs.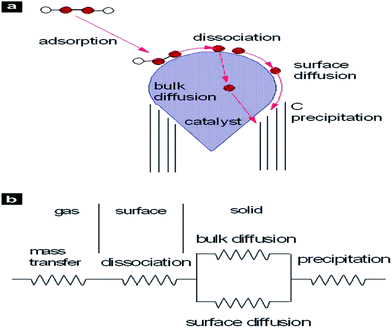 | ||
| Fig. 2 (a) Schematic of the CNT growth process, (b) schematic of possible rate-limiting processes. Reproduced with permission from ref. 30. | ||
For several decades, the decomposition or reduction of CO2 over metal substrates has been studied for industrial processes and pollution control.33–35 The catalysed or un-catalysed thermal splitting of CO2 involves the following overall reaction:36
| CO2 → C + O2 | (1) |
However, the initial stage in the splitting of CO2 produces carbon monoxide (CO) and oxygen18,36,37 as shown in reaction (2).
| 2CO2 → 2CO + O2 | (2) |
After reaction (2), the two reactions which merit consideration as possible means for the complete splitting process are either via the Boudouard reaction which involves the disproportionation of CO to carbon and CO2 (see reaction (3)) or direct splitting of CO to carbon and oxygen as given by reaction (4).
| 2CO → C + CO2 | (3) |
| CO → C + 1/2O2 | (4) |
However, the reaction represented by eqn (4) can be ruled out because it is only likely to occur at extremely high temperatures.36 Therefore, reaction (3) is the only one considered here, and this reaction on iron catalyst has long been known to produce filamentous carbon;38 such filaments with a tubular graphic structure later came to be called CNTs.39 In fact, CO has now become one of the common carbon sources for the production of CNTs40–43 due to the reasonable temperature range for CO disproportionation and the good CNT yield.44 Modelling of CNT production from CO is rare except for the numerical simulations of a HiPco floating catalyst CVD reactor.40,45
There are a number of possible elementary reaction processes occurring before the production of CNTs. Initially, CO2 adsorbs on the catalyst surface,
| CO2(gas) ↔ CO2(ads) | (5) |
| CO2(ads) ↔ CO(ads) + O(ads) | (6) |
The reaction rates for reaction (6) can be calculated for the forward and reverse reactions as,
| Rforward = k1[CO2(ads)] | (7) |
| Rreverse = k−1[CO(ads)][O(ads)] | (8) |
The adsorbed CO in reaction (6) first dissociates into carbon and oxygen, and then, the adsorbed oxygen reacts with the undissociated CO to form surface or adsorbed44 CO2 which desorbs to gaseous CO2 as shown in reactions (9), (10), and (11), respectively.
| CO(ads) ↔ C(ads) + O(ads) | (9) |
| CO(ads) + O(ads) ↔ CO2(ads) | (10) |
| CO2(ads) ↔ CO2(gas) | (11) |
In order to obtain the rate equation for the Boudouard reaction, reactions (9) and (10) are first combined as overall reaction (12),
| 2CO(ads) ↔ C(ads) + CO2(ads) | (12) |
The rate constants for the forward and reverse reactions (12) of the equilibrium are given as k2 and k−2 in eqn (13) and (14), respectively,
| Rforward = k2[CO(ads)]2 | (13) |
| Rreverse = k−2[C(ads)][CO2(ads)] | (14) |
As shown in Fig. 2, the carbon species produced in reaction (12) diffuse into or on the surface of the catalyst particles, nucleate and segregate to the surface forming the graphene networks of CNTs,
| C(ads) → CNT | (15) |
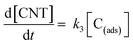 | (16) |
In eqn (16), k3 is the rate constant proportional to the diffusion coefficient (or rate) of carbons in the metal catalyst nanoparticles.46 If the rates of formation of the intermediate products and their decay back into reactants were much faster than the rate of formation of CNTs (k1 ≫ k3; k2 ≫ k3), then the rate-determining step of CNT growth would be the reaction (15). In other words, reactions (5), (6), (9) and (10) are fast reactions followed by a slower reaction of carbon diffusing through (or on the) solid catalyst and precipitating to form the nanotube. Since it is assumed that the reactants are in equilibrium with the intermediate products, the equilibrium constants, K1 and K2, are then defined as follows:
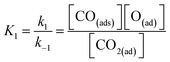 | (17) |
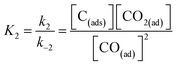 | (18) |
Therefore, the growth rate of CNTs is given as follows:
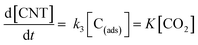 | (19) |
H2 was used in this work together with CO2 as mentioned in the materials and methods section. Nasibulin and colleagues have already demonstrated the important role of H2 in the CVD process.43 However, the following needs to be noted: H2 is believed to prevent the oxidation of the nanosized catalyst iron particles, thus maintaining the catalyst in their reduced state.43,44,49 In fact, Moisala et al.50 thermodynamically showed that only non-oxide metal particles behave as catalysts in the CNT formation, thus metal oxide particles must be reduced. In addition, H2 limits or alleviates the carbon poisoning of the metal catalysts.51 It has also been reported that adsorbed H2 on the catalyst surface catalyses the disproportionation52 of CO. Though not related to this study, H2 reduces the rate of dehydrogenation of the CHx intermediates which are responsible for soot formation.53 Finally, at the studied furnace temperature, H2 can provide additional carbon atoms for the CNT synthesis from its reaction with CO2 under the temperature conditions of this study as shown in eqn (20) below.18,44
| CO2 + 2H2 ↔ 2H2O + C | (20) |
However, the reaction with CNTs and hydrogen, as given by reaction (21),
| 2C + 4H2 ↔ 2CH4 | (21) |
Results and discussion
Identification of CNTs
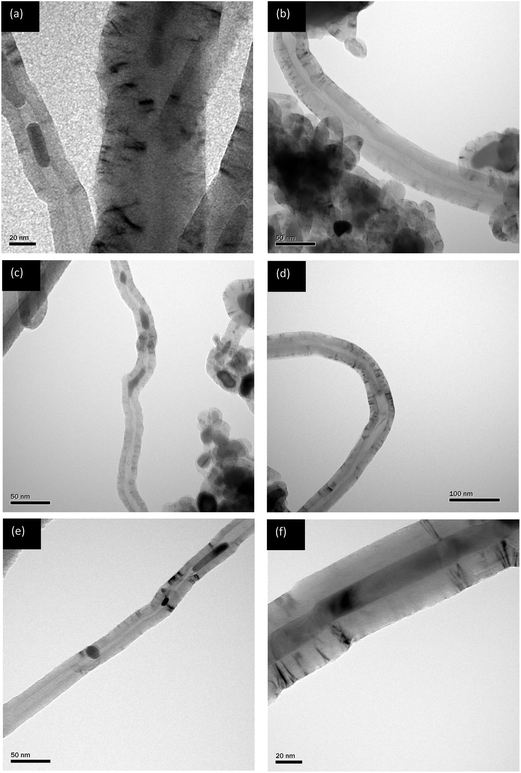
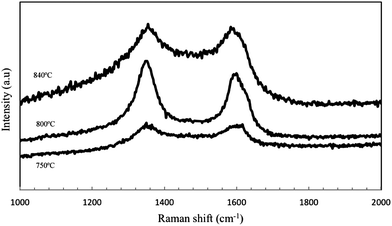
The TEM images in Fig. 3 confirms that the deposited carbons are CNTs, produced at temperatures 750, 800 and 840 °C. The filamentous carbon possesses hollow cores without any bamboo-like closures and the diameter distribution of the as-produced CNTs is rather uniform with an average diameter less than 100 nm. Fig. 4 is the Raman spectra of the as-grown CNTs shown also for growth temperatures of 750, 800 and 840 °C. The basis for the Raman spectra revolves around the assignment of the G-band (graphitic, ∼1580 cm−1) to crystalline graphite and any other bands, called D-bands (disorder, varies from 1100 to 1500 cm−1) to any type of structural disorder in the graphitic structure.54 However, the other band that is characteristic only for single-walled CNTs called radial breathing mode (RBM) lie in the region55 of 100–400 cm−1. Fig. 4 shows two significant peaks at ∼1358 cm−1 and ∼1582 cm−1, representing the D-band and G-band, respectively. As stated already, the G-band represents the graphitisation degree of the nanotube structure while the D-band describes the degree of structural defects including amorphous (non-graphitic) carbon adhered on the nanotube structure.56,57 The absence of the RBM in the Raman spectra implies that the CNTs produced are multi-walled.The ID/IG ratio, which shows the relative intensity of the D- and G-bands of a Raman spectrum and an indicator of the degree of graphitization and crystallinity,57,58 has ratios of 0.99, 1.08 and 1.02 for temperatures of 750, 800 and 840 °C respectively. These values suggest that the as-produced CNTs exhibit a remarkable crystallinity as the temperature increases, implying that the raw CNTs have smoother carbon surfaces and less structural defects. These results agree with the TEM images in Fig. 3. In general, the purity of CNTs produced in this study is high and the higher purity of CNTs from CO2 relative to those produced from gaseous hydrocarbons was also reported by Xu and Huang.18
Comparison of kinetic model with experimental data
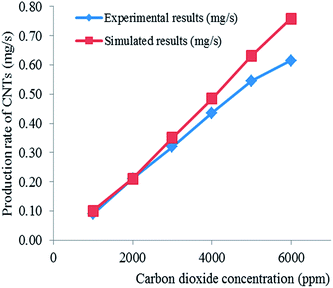
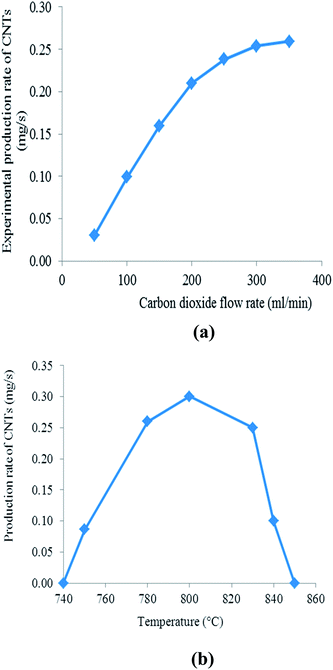
The derived kinetic model can be used to calculate the amount or yield of CNTs for a given volumetric flow rate (or concentration) of CO2. A comparison of the simulated results and the experimental as well as the general effects (not based on the model, but on the actual observations) of other parameters on the production of CNTs are discussed below.The CO disproportionation reaction (eqn (3) or (12)) has two main limitations, namely: (1) the reaction is limited kinetically at lower temperatures, and (2) it is limited thermodynamically at higher temperatures.43 Previous studies have shown that kinetic and thermodynamic factors limit the effective CO disproportionation reaction to a temperature range of 520–800 °C at atmospheric pressure.50 Equilibrium favours the right side65 at temperatures below 700 °C. The observations by Moisala et al.50 and Boehm65 agree with the results of this study which show an increase in CNT production for temperatures lower than about 800 °C. However, attainment of equilibrium is possible only in the presence of catalysts, mainly iron, cobalt, and nickel. Even then, temperatures in excess of 400 °C are needed for measurable reaction rates, with maximum rates observed65 around 550 °C. Furthermore, previous studies have also shown that although the conversion of CO may be fast at lower temperatures, the CNT yield is very low.66 This is because the plentiful amount of carbon that forms on the catalyst surface is not able to be transferred away in time due to the relatively low rate of carbon diffusion in the bulk of the metal catalyst particles at low temperatures, and thus, blocks the active catalyst surface leading to a decrease in activity.66
In summary, CO2 decomposition is an endothermic reaction with CO formation being favoured at higher temperatures. In contrast, CO disproportionation is an exothermic reaction, such that both the CO conversion and carbon formation decline as the reaction temperature is increased. In addition, at higher temperatures, CO2 acting as a mild oxidising agent can react with the graphitic layers of the CNTs, leading to the erosion of CNTs and thus, a low yield is achieved.67 There are no oxidation reactions for CNTs at lower temperatures.68
Since the Boudouard reaction is an exothermic reaction limited by equilibrium at the high temperatures needed to activate CO on the catalyst, it has been found that high CO partial pressures are needed in order to counter the effect of temperature and drive the reaction in the forward direction.43,69,70 Therefore, high pressure results in both the enhancement of the reaction rate due to an increase in the amount of reagent and in the widening of the temperature range for CNT production.43
Importance of further kinetic model development for large-scale CNT synthesis
Mass production of CNTs continues to be a challenge and though the literature has been vast and growing, kinetic models are scarce. Table 1 outlines the limited kinetic models available and the limited number of studies has an impact on our understanding and the consequent slow progress. The use of pure gases (hydrocarbons or otherwise) as feedstock is very expensive as they are limited in supply. Moreover, most organic gases are toxic and difficult to store and transport. The precursor materials for bulk production of CNTs should be economically attractive, available in plentiful supply and easy to pyrolyze.7 Studies have shown that CNTs can be successfully produced from liquefied petroleum gas,75 natural gas,76 coal gas77,78 and directly from coal.79 However, more experimental work is needed to improve the yield and quality of CNTs produced from these ‘impure’ carbon sources. A major challenge in using these impure sources is that complete study of the species present in the gas phase at high temperature is difficult to achieve, as decomposition of the carbon precursor involves many steps. Additionally, the recombination and reactivity of the initial species make it difficult to know which species are in fact the true gas phase intermediates that actively take part in the growth of CNTs.80 As a result, the possible synergic effect of gaseous species in coal gas that results in CNT synthesis is unclear.77,78 In order to fulfill this requirement, the kinetic model (eqn (19)) developed for CNT production from CO2 needs to be modified to make the provisional first steps in understanding these possible effects.| Carbon Source | Catalyst | Proposed model for CNT production | Ref. |
|---|---|---|---|
| Carbon monoxide (CO) | Fe(CO)5 | 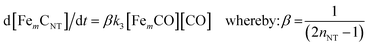 |
40,45 |
| Acetylene (C2H2) | Iron supported (Fe/silica) | 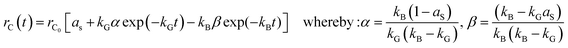 |
71 |
| Acetylene (C2H2) | Ferrocene | 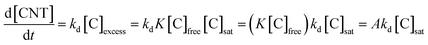 |
46 |
| Acetylene (C2H2) | Ni- and Co-supported CaCO3 | 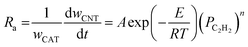 |
72,73 |
| Ethylene (C2H4) | Fe/Al2O3 | 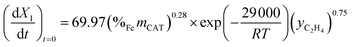 |
74 |
| Carbon dioxide (CO2) | Fe-supported CaCO3 | 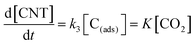 |
This study |
Conclusions
The combination of the potential decomposition mechanisms of CO2 and carbon diffusion was utilised to formulate a simple kinetic model for the production of CNTs. The results illustrate the importance of including the thermal decomposition step. There is no well-known kinetic model to date for the production of CNTs from CO2 and the kinetic model derived here was used to calculate the amount of CNTs at various concentrations of CO2. The experimentally measured production rate data fits the simple rate equation very well at low CO2 concentrations. The analysis here can be extended to other carbon sources used in the production of CNTs. The influence of CO2 flow rate was also studied. Just like concentration, very high flow rates adversely affect the CNT growth rates indicating the importance of controlling the concentration and flow rate of the carbon source. The results also show that the temperature plays an important role in the synthesis of CNTs from CO2: when the temperature is lower than 750 °C or above 840 °C, no CNTs are formed and the optimum growth temperature is about 800 °C.Acknowledgements
The authors wish to thank Dr Lubinda F. Walubita of TTI – The Texas A&M University System (USA) for his insightful technical comments on the paper. The financial support from the National Research Foundation (NRF) under South Africa NRF Focus Area, NRF Nanotechnology flagship programme, DST/NRF Centre of Excellence and University of the Witwatersrand Staff Bursary is gratefully acknowledged.References
- M. Aresta, Stud. Surf. Sci. Catal., 1998, 114, 65–76 CrossRef CAS.
- Q. W. Chen and D. W. Bahnemann, J. Am. Chem. Soc., 2000, 122, 970–971 CrossRef CAS.
- G. A. Olah, A. Goeppert and G. K. S. Prakash, J. Org. Chem., 2009, 74, 487–498 CrossRef CAS PubMed.
- F. T. Zangeneh, S. Sahebdelfar and M. T. Ravanchi, J. Nat. Gas Chem., 2011, 20, 219–231 CrossRef CAS.
- G. S. Simate, S. E. Iyuke, S. Ndlovu, C. S. Yah and L. F. Walubita, J. Nat. Gas Chem., 2010, 19, 453–460 CrossRef CAS.
- M. Meyyappan, Carbon Nanotubes: Science and Applications, Boca Raton, FL, CRC Press, 2004 Search PubMed.
- K. Dasgupta, J. B. Joshi and S. Banerjee, Chem. Eng. J., 2011, 171, 841–869 CrossRef CAS.
- S. E. Iyuke and A. B. M. Danna, Microporous Mesoporous Mater., 2005, 84, 338–342 CrossRef CAS.
- A. S. Afolabi, A. S. Abdulkareem and S. E. Iyuke, J. Exp. Nanosci., 2007, 2, 269–277 CrossRef CAS.
- S. E. Iyuke, T. A. Mamvura, K. Liu, V. Sibanda, M. Meyyappan and V. K. Varadan, Nanotechnology, 2009, 20, 375602–375611 CrossRef CAS PubMed.
- N. M. Mohamed and L. M. Kou, J. Appl. Sci., 2011, 11, 1341–1345 CrossRef CAS.
- J. M. Ngoy, S. E. Iyuke, W. E. Neuse and C. S. Yah, J. Appl. Sci., 2011, 11, 2700–2711 CrossRef CAS.
- L. Fekri, A. Jafari, S. Fekri, A. Shafikhani, M. Vesaghi and G. Behzadi, J. Appl. Sci., 2010, 10, 716–723 CrossRef CAS.
- M. Khavarian, S. P. Chai, S. H. Tan and A. R. Mohamed, J. Appl. Sci., 2011, 11, 2382–2387 CrossRef CAS.
- E. Mora, T. Tokune and A. R. Harutyunyan, Carbon, 2007, 45, 971–977 CrossRef CAS.
- A. S. Anisimov, A. G. Nasibulin, H. Jiang, P. Launois, J. Cambedouzou and S. D. Shandakov, Carbon, 2010, 48, 380–388 CrossRef CAS.
- L. Ci, Y. Li, B. Wei, J. Liang, C. Xu and D. Wu, Carbon, 2000, 38, 1933–1937 CrossRef CAS.
- X.-J. Xu and S.-M. Huang, Mater. Lett., 2007, 61, 4235–4237 CrossRef CAS.
- S. D. Mhlanga, Synthesis and study of carbon nanotubes and carbon spheres, PhD thesis, Johannesburg, University of the Witwatersrand, 2009.
- C. H. See and A. T. Harris, AIChE J., 2008, 54, 657–664 CrossRef CAS.
- Z. Li, E. Dervishi, Y. Xu, V. Saini and M. Mahmood, Catal. Lett., 2009, 131, 356–363 CrossRef CAS.
- C. S. Yah, S. E. Iyuke, G. S. Simate, E. I. Unuabonah, G. Bathgate and G. Matthews, J. Mater. Res., 2011, 26, 640–644 CrossRef CAS.
- C. S. Yah, G. S. Simate, K. Moothi, S. K. Maphutha and S. E. Iyuke, Trends Appl. Sci. Res., 2011, 6, 1270–1279 CrossRef CAS.
- A. Thess, R. Lee, P. Nikolaev, H. Dai, P. Petit, J. Robert and C. Xu, Science, 1996, 273, 483–487 CAS.
- Y. H. Lee, S. G. Kim and D. Tomanek, Phys. Rev. Lett., 1997, 78, 2393–2396 CrossRef CAS.
- M. Yudasaka, R. Yamada, N. Sensui, T. Wilkins, T. Ichihashi and S. Iijima, J. Phys. Chem. B, 1999, 103, 6224–6229 CrossRef CAS.
- H. Kataura, Y. Kumazawa, Y. Maniwa, Y. Ohtsuka, R. Sen and S. Suzuki, Carbon, 2000, 38, 1691–1697 CrossRef CAS.
- Y. Saito, Carbon, 1995, 33, 979–988 CrossRef CAS.
- K. Raji, S. Thomas and C. B. Sobhan, Appl. Surf. Sci., 2011, 257, 10562–10570 CrossRef CAS.
- C. T. Wirth, C. Zhang, G. Zhong, S. Hofmann and J. Robertson, ACS Nano, 2009, 3, 3560–3566 CrossRef CAS PubMed.
- S. Hofmann, G. Csanyi, A. C. Ferrari, M. C. Payne and J. Robertson, Phys. Rev. Lett., 2005, 95, 036101 CrossRef CAS PubMed.
- O. Sharia and M. M. Kuklja, J. Phys. Chem. A, 2010, 114, 12656–12661 CrossRef CAS PubMed.
- R. G. Copperthwaite, P. R. Davies, M. A. Morris, M. W. Roberts and R. A. Ryder, Catal. Lett., 1988, 1, 11–19 CrossRef CAS.
- H. Kato, T. Kodama, M. Tsuji, Y. Tamaura and S. C. Chang, J. Mater. Sci., 1994, 29, 5689–5692 CrossRef CAS.
- Z. Chun-lei, L. Zhi-qiang, W. Tong-hao, Y. Hong-mao, J. Yu-zi and P. Shao-yi, Mater. Chem. Phys., 1996, 44, 194–198 CrossRef.
- S. Rayne, Thermal carbon dioxide splitting: A summary of the peer-reviewed scientific literature, Nature Precedings, 2008, DOI:10.1038/npre.2008.1741.2.
- H. C. Shin, S. C. Choi, K. D. Jung and S. H. Han, Chem. Mater., 2001, 13, 1238–1242 CrossRef CAS.
- M. Monthioux and V. L. Kuznetsov, Carbon, 2006, 44, 1621–1623 CrossRef CAS.
- S. Iijima, Nature, 1991, 354, 56–58 CrossRef CAS.
- C. Dateo, T. Gokcen and M. Meyyappan, J. Nanosci. Nanotechnol., 2002, 2, 523–534 CrossRef CAS PubMed.
- A. G. Nasibulin, A. Moisala, D. P. Brown and E. I. Kauppinen, Carbon, 2003, 41, 2711–2724 CrossRef CAS.
- A. Moisala, A. G. Nasibulin, D. P. Brown, H. Jiang, L. Khriachtchev and E. I. Kauppinen, Chem. Eng. Sci., 2006, 61, 4393–4402 CrossRef CAS.
- A. G. Nasibulin, P. Queipo, S. D. Shandakov, D. P. Brown, H. Jiang and P. V. Pikhitsa, J. Nanosci. Nanotechnol., 2006, 6, 1233–1246 CrossRef CAS PubMed.
- G. Lanzani, A. G. Nasibulin, K. Laasonen and E. I. Kauppinen, Nano Res., 2009, 2, 660–670 CrossRef CAS.
- T. Gokcen, C. Dateo and M. Meyyappan, J. Nanosci. Nanotechnol., 2002, 2, 535–544 CrossRef CAS PubMed.
- K. E. Kim, K. J. Kim, W. S. Jung, S. Y. Bae, J. Park and J. Choi, Chem. Phys. Lett., 2005, 401, 459–464 CrossRef CAS.
- R. T. K. Baker, Carbon, 1989, 27, 315–323 CrossRef CAS.
- E. A. Brandes and G. B. Book, Smithells Metals Reference Book, London, Butterworth-Heinemann Limited, 7th edn, 1992 Search PubMed.
- M. C. Bahome, L. L. Jewell, D. Hildebrandt, D. Glasser and N. J. Coville, Appl. Catal., A, 2005, 287, 60–67 CrossRef CAS.
- A. Moisala, A. G. Nasibulin and E. I. Kauppinen, J. Phys.: Condens. Matter, 2003, 15, S3011–S3035 CrossRef CAS.
- M. S. Kim, N. M. Rodriguez and R. T. K. Baker, J. Catal., 1991, 131, 60–73 CrossRef CAS.
- B. Zheng, C. Lu, G. Gu, A. Makarovski, G. Finkelstein and J. Liu, Nano Lett., 2002, 2, 895–898 CrossRef CAS.
- P. T. A. Reilly and W. B. Whitten, Carbon, 2006, 44, 1653–1660 CrossRef CAS.
- S. Potgieter-Vermaak, N. Maledi, N. Wagner, J. H. P. van Heerden, R. van Grieken and J. H. J. Potgieter, Raman Spectrosc., 2011, 42, 123–129 CrossRef CAS.
- S. Osswald, M. Havel and Y. J. Gogotsi, Raman Spectrosc., 2007, 38, 728–736 CrossRef CAS.
- S. P. Patole, P. S. Alegaonkar, J. H. Lee and J. B. Yoo, Europhys. Lett., 2008, 81, 1–6 CrossRef.
- K. Y. Lee, W. M. Yeoha, S. P. Chaib, S. Ichikawac and A. R. Mohameda, Fullerenes, Nanotubes, Carbon Nanostruct., 2010, 18, 273–284 CrossRef CAS.
- T. Tsoufis, P. Xidas, L. Jankovic, D. Gournis, A. Saranti and T. Bakas, Diamond Relat. Mater., 2007, 16, 155–160 CrossRef CAS.
- R. Andrews, D. Jacques, A. M. Rao, F. Derbyshire, D. Qian and X. Fan, Chem. Phys. Lett., 1999, 303, 467–474 CrossRef CAS.
- X. Y. Li, B. C. Huang and N. J. Coville, Fullerenes, Nanotubes, Carbon Nanostruct., 2002, 10, 339–352 CrossRef.
- R. M. M. Abbaslou, J. Soltan and A. K. Dalai, Appl. Catal., A, 2010, 372, 147–152 CrossRef.
- C. Singh, M. S. P. Shaffer and A. H. Windle, Carbon, 2003, 41, 359–368 CrossRef CAS.
- S. M. Toussi, A. Fakhru'l-Razi, A. L. Chuah and A. R. Suraya, Mater. Sci. Eng., 2011, 17, 012003 Search PubMed.
- C. Vallés, M. Pérez-Mendoza, W. K. Maser, M. T. Martínez, L. Alvarez and J. L. Sauvajol, Carbon, 2009, 47, 998–1004 CrossRef.
- H. P. Boehm, Carbon, 1973, 11, 583–536 CrossRef.
- P. Chen, H. B. Zhang, G. D. Lin, Q. Hong and K. R. Tsai, Carbon, 1997, 35, 1495–1501 CrossRef CAS.
- S. C. Tsang, P. J. F. Harris and M. L. H. Green, Nature, 1993, 362, 520–522 CrossRef CAS.
- Z. Lou, C. Chen, Q. Chen and J. Gao, Carbon, 2005, 43, 1104–1108 CrossRef CAS.
- P. Nikolaev, M. J. Bronikowski, R. K. Bradley, F. Rohmund, D. T. Colbert and K. A. Smith, Chem. Phys. Lett., 1999, 313, 91–97 CrossRef CAS.
- D. E. Resasco, W. E. Alvarez, F. Pompeo, L. Balzano, J. E. Herrera and B. Kitiyanan, J. Nanopart. Res., 2002, 4, 131–136 CrossRef CAS.
- M. P. Cabero, E. Romeo, C. Royo, A. Monzon, A. G. Ruiz and I. Rodríguez-Ramos, J. Catal., 2004, 224, 197–205 CrossRef.
- C. T. Hsieh, Y. T. Lin, J. Y. Lin and J. L. Wei, Chem. Phys., 2009, 114, 702–708 CAS.
- C. T. Hsieh, Y. T. Lin, W. Y. Chen and J. L. Wei, Powder Technol., 2009, 192, 16–22 CrossRef CAS.
- R. Philippe, P. Serp, P. Kalck, Y. Kihn, S. Borde're and D. Plee, AIChE J., 2009, 55, 450–464 CrossRef CAS.
- W. Qian, H. Yu, F. Wei, Q. Zhang and Z. Wang, Carbon, 2002, 40, 2961–2973 CrossRef.
- R. Bonadiman, M. D. Lima, M. J. de Andrade and C. P. Bergmann, J. Mater. Sci., 2006, 41, 7288–7295 CrossRef CAS.
- J. Qiu, Y. An, Z. Zhao, Y. Li and Y. Zhou, Fuel Process. Technol., 2004, 85, 913–920 CrossRef CAS.
- J. Qiu, Q. Li, Z. Wang, Y. Sun and H. Zhang, Carbon, 2006, 44, 2565–2568 CrossRef CAS.
- K. Moothi, S. Iyuke, M. Meyyappan and R. Falcon, Carbon, 2012, 50, 2679–2690 CrossRef CAS.
- A. Shaikjee and N. J. Coville, Carbon, 2012, 50, 3376–3398 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |