Synthesis of new polyethoxylated tertiary amines and their use as Switchable Hydrophilicity Solvents†
Chiara Samorì*a,
Laura Pezzolesia,
Diego López Barreirob,
Paola Gallettia,
Andrea Pasterisa and
Emilio Tagliavinia
aCentro Interdipartimentale di Ricerca Industriale (CIRI), University of Bologna, via S. Alberto 163, Ravenna, Italy. E-mail: chiara.samori3@unibo.it; Fax: +39-0544-937411; Tel: +39-0544-937353
bDepartment of Biosystems Engineering, University of Ghent, Ghent, Belgium
First published on 18th December 2013
Abstract
New polyethoxylated tertiary amines were synthesised and applied as Switchable Hydrophilicity Solvents (SHS) in the extraction and recovery of lipids from algal cultures, bypassing harvesting and de-watering steps. The eco-toxicological profile of the new amines was explored by evaluating their potential in inhibiting algal growth, and deepened by measuring the acute toxicity towards the crustacean Daphnia magna and the ready biodegradability in water. Among the synthesised amines, the one bearing two ethoxy units, named amine 2, showed the best combination in terms of lipids' extractive performances and toxicity; therefore it could be considered as a good alternative to N,N-dimethylcyclohexylamine (DMCHA), at the moment the most studied and effective SHS.
Introduction
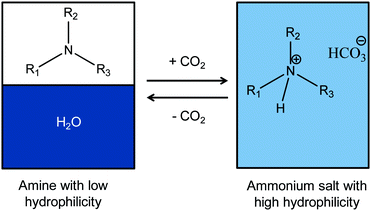
Switchable polarity solvents (SPS) are a fascinating class of extracting media, capable of turning from a non-ionic apolar form to an ionic liquid by simply adding and removing CO2.1 In the last decade the potential applicability of switchable systems has begun to be widely explored:2 switchable surfactants,3,4 switchable media for polymerizations5 and catalytic reactions,6 switchable extraction systems,7,8 represent just some of the many examples which have been successfully developed. In particular, Jessop's group and our group have used SPS systems for the obtainment of biofuels from microalgae, widening the potential applications of these systems as green and sustainable technologies.9–12 The extraction of lipids from microalgal biomass is a challenging task, critical in the overall economic balance of biodiesel production: the development of new efficient extraction systems, directly applicable to algal cultures by-passing concentration and dewatering steps, could support a breakthrough towards the sustainable production of third generation biodiesel.Among SPS some tertiary amines, like N,N-dimethylcyclohexylamine (DMCHA), have a tuneable hydrophilicity, thus being also called Switchable Hydrophilicity Solvents (SHS). This means that they can be switched from hydrophobic neutral forms (with a low water-solubility), into water-soluble hydrophilic hydrogen carbonate ammonium salts by adding CO2 (Fig. 1).13
As stated by Jessop et al.,13 the number of commercially available tertiary amines suitable as “green and safe” SHS systems for extraction processes is restricted to a narrow group of compounds. Among them, DMCHA appeared to be the best option, considering the following features expected for a “good” SHS amine which should: (i) be immiscible with water (in the neutral form) and become miscible with water upon switching with CO2; (ii) be liquid at room temperature (in the neutral form); (iii) have a high boiling and (iv) have a low toxicity. For example, according to these four points, tertiary alkanolamines (e.g. triethanolamine, TEA), widely used for CO2-capture in industrial plants, are not suitable as SHS due to the high water-solubility of the neutral form, in spite of being highly biodegradable and poorly toxic towards aquatic species.14
Our main goal in the present paper is widening the pool of SHS-suitable tertiary amines by synthesising new candidates which can be used as alternative to DMCHA.
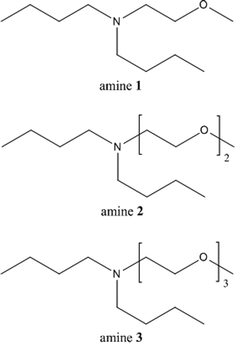
In our previous studies on the reduction of ionic liquids' eco-toxicity by introducing (poly)ethoxy moieties in the cation structure,15–17 we have demonstrated that, as a general trend, the substitution of a purely alkyl lateral chain with a (poly)ethoxylated one on the imidazolinium core of ionic liquids drastically reduces the adverse effects of these compounds at different levels of biological organization. We have attributed this behavior to a decrease in the lipophilicity of ionic liquids, with a consequent reduction of their interactions with cellular membranes. Taking advantage of these previous studies, we have designed and synthesised three new oxygenated tertiary amines starting from dibutylamine: N-(methoxyethyl)dibutylamine 1, N-(methoxyethoxyethyl)dibutylamine 2 and N-(methoxydiethoxyethyl)dibutylamine 3 (Fig. 2).
The new amines 1, 2 and 3 were characterized in terms of solubility in water, hydrolytic stability and “switchability”. Also, in order to determine the applicability in a SHS extraction system, their efficiency in the extraction and recovery of algal lipids was tested on the marine microalga Nannochloropsis gaditana, well known for its ability to produce high level of triacylglycerols. In the field of third generation biofuels, one of the most challenging task is the reduction of the economic and energy costs associated to algal cultivation; specifically, significant improvements in this sense could be obtained if the aqueous phase would be recycled for growing algae again, for example after any extraction/downstream process, allowing the recycling of the nutrients (N and P). In the case of lipid extraction through SHS, the possibility of recycling the algal growth medium is strictly regulated by (i) the loss of amine in the water phase and (ii) its toxicity towards algae. Thus, for clarifying these two points and for deepening the environmental impact of the three amines we checked (i) algal growth inhibition by using N. gaditana as target organism; (ii) biodegradability in water and (iii) acute toxicity towards the crustacean Daphnia magna. These aspects cover the basic required information necessary for REACH registration (regulation (EC) No 1907/2006 of the European Union).
Results and discussion
The new amines were prepared in very high overall yields (87%, 91% and 95% for 1, 2 and 3, respectively) by alkylation of dibutylamine with the corresponding alkyl chlorides under solvent-free conditions (Fig. 3).All of them have a low vapor pressure, are highly boiling and poorly miscible with water (Table 1); their solubility is lower than that of DMCHA (18 g L−1) and similar to that of their precursor, dibutylamine (4.05 g L−1); the water solubility increases by increasing the number of ethoxy units in the chemical structure. Each amine shows a “switchable” behavior upon the addition of CO2: they constitute a biphasic mixture with water when they are in the neutral form, which is turned into a homogeneous phase upon addition of CO2 for 45 min (Fig. 4), forming ammonium salts 8, 9, 10, completely water-soluble; the resulting solutions of ammonium salts 8, 9 and 10 can be reversibly switched back into amines 1, 2 and 3, respectively, by stirring the solution at 80 °C, allowing CO2 removal.
| Amine | Water solubility (g L−1) | Boiling point (°C) | Vapor pressure (atm at 25 °C) |
|---|---|---|---|
| 1 | 1.8 | 225 | 1.3 × 10−4 |
| 2 | 4.5 | 277 | 5.6 × 10−5 |
| 3 | 5.9 | 322 | 2.4 × 10−7 |
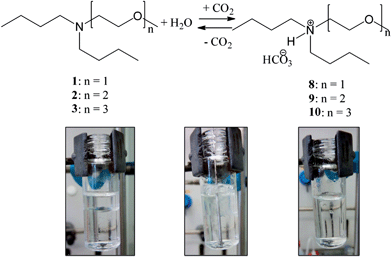 | ||
| Fig. 4 Reversible switching of amines 1, 2 and 3 into hydrogen carbonates ammonium salts 8, 9 and 10 by the addition and the removal of CO2. | ||
The fact that our new amines 1, 2, and 3 have a reversible miscibility with water makes them suitable for being used as SHS in extraction processes of low-polarity compounds, such as lipids, from aqueous systems like algal cultures, analogously to DMCHA.11 As mentioned before, the possibility of recycling the water phase for re-growing algae after the extraction is related to the solubility/losses of the amine in water and to its toxicity; the water solubility of DMCHA is 18 g L−1, whereas its algal toxicity is around three orders of magnitude lower than this value (e.g. EC50 towards the green alga Desmodesmus subspicatus is 88.5 mg L−1).18 This means that in order to reuse the water phase, it should be diluted at least 1000-fold. Since the three new tertiary amines here synthesized have a lower water solubility than DMCHA, the expected losses in water should be lower; moreover, since we expect a low (eco)-toxicity, we think that they could be a better solution then DMCHA when the issue of recycling the algal culture medium is taken into account.
Lipid extraction efficiency
Algal oils typically contain a complex mixture of chemical compounds with a wide range of polarity, of which only a small amount, usually represented by fatty acids in their free and bounded (triacylglycerols) form, is exploitable in biofuel applications. Algal extracts obtained with chloroform/methanol, for example, are composed of hydrocarbons, phytols, triacylglycerols, free fatty acids, phospholipids, hydrophobic protein derivatives and other nitrogen compounds, alcohols, and pigments. Thus for the obtainment of third generation biodiesel from algal oils, a series of steps of purification and de-acidification are needed.19In the present paper we focused our attention on the development of SHS directly applicable to the extraction of algal cultures, with the aim of reducing the costs related to algae treatments because of low energy consumption: by using a SHS system in fact no energy is used for de-watering the biomass, the extractions are performed at room temperature, the algal lipids are recovered by the addition of CO2 and the solvent system is recycled (switched-back) by CO2 removal. Thus, after having verified that amines 1, 2, and 3 have a good switchable hydrophilicity behavior, the other condition necessary for using them in extracting switchable hydrophilicity systems is testing their performances as suitable solvents for recovery lipids from algal cultures.
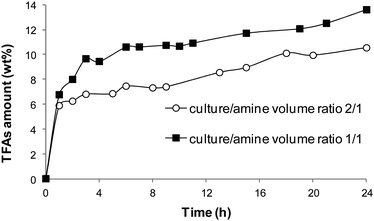
The efficiency of amines 1, 2 and 3 as media for extracting N. gaditana cultures with a biomass concentration of around 2 g L−1 was tested with two culture/amine volume ratios: 2/1 (algal biomass/amine ratios of 4 mg mL−1, Fig. 5a) and 1/1 (algal biomass/amine ratios of 2 mg mL−1, Fig. 5b). The extraction efficiency has been reported as capability of extracting biodiesel-like compounds (bounded and free fatty acids, BFAs and FFAs, respectively) from algal cells. The sum of BFAs and FFAs represents the total fatty acid (TFAs) content of the alga; TFAs were determined as fatty acid methyl esters (FAMEs) after the conversion of BFAs and FFAs into FAMEs through an analytical esterification/transesterification procedure by using NaOH/MeOH and BF3-methanol (see the Experimental part for details).
It can be seen that in the system with the ratio 2/1 (Fig. 5a), all the tested amines are not able to completely extract all the biodiesel-like compounds of the alga, affording an amount of TFAs lower than the total fatty acids initially present in the biomass (15 wt%, extracted from dried biomass with CHCl3/MeOH under reflux; the red dotted line in Fig. 5a and b). The three amines have a similar extracting capacity and the TFAs amounts obtained after 24 h are almost equal (8.4 ± 0.9 wt% with amine 1, 8.9 ± 0.5 wt% with amine 2 and 9.5 ± 1.0 wt% with amine 3). These amounts are slightly lower than what obtained by using DMCHA under the same conditions (11.9 ± 1.5 wt%).11 By increasing the amount of amine per volume of culture, the biodiesel-like compounds' extraction efficiency increases for all the amines: amine 3 gives the best results after 24 h with a TFAs extracted content of 14.6 ± 1.8 wt%, followed by amine 2 (12.7 ± 0.1 wt%) and amine 1 (11.5 ± 1.9 wt%), both of them comparable to DMCHA (TFAs amount of 10.8 ± 0.1 wt%).11
Algal growth inhibition tests
The recycling of the water phase after a lipid extraction process is an important task in order to reduce the consumption of nutrients and water; however if the loss of the solvent used for the extraction in the water phase is significant and if the solvent is highly toxic for algae, the recycle could result problematic. Thus, in order to get more information about the new SHS systems based on amines 1, 2 and 3 here synthesised, the effects of the three amines here synthesized and DMCHA on the growth of N. gaditana were estimated with respect to control samples (normalized response), through a 72 h growth inhibition test. For adapting this toxicity measure to the here-proposed process of lipid-extraction with SHS, each amine was kept in contact with algal growth medium for 24 h, simulating the duration of the extraction process from N. gaditana cultures previously described (Fig. 5); after that, the amine layer was withdrawn and the water phase was diluted to get each specific concentration used in the toxicity test.The EC50 values are shown in Table 2.
| Amine | EC50 (mg L−1) |
|---|---|
| 1 | 22 (15–33) |
| 2 | 383 (345–425) |
| 3 | 47 (37–60) |
| DMCHA | 271 (231–318) |
The EC50 values vary a lot among the amines, ranging from 22 to 383 mg L−1, indicating that compound 1 is the most toxic among the tested amines and 2 is the least.
Surprisingly, amines 1 and 3 show a totally different behaviour with respect to amine 2, being about 10-fold more toxic; moreover both of them are more toxic than DMCHA. To understand this unexpected result, we investigated the hypothesis that the amount of amines which is effectively present in water (according to Table 1, it is 1.8, 4.5 and 5.9 g L−1 for amine 1, 2 and 3, respectively) undergoes to a hydrolytic process during the 72 h of duration of the test, giving rise to the formation of highly-toxic by-products. Therefore the stability of the three amines was assessed in deionized water, freshly-prepared algal growth medium (without algae) and algal growth medium containing algae (Fig. 6): specifically, each amine was taking in contact with each water phase for 24 h for equilibrating the biphasic system; after that, the amine layer was removed and the aqueous solutions, containing an amount of each amine corresponding to each specific water solubility (see Table 1), were stirred for 72 h (to simulate the duration of the toxicity test) and then analysed.
 | ||
| Fig. 6 Stability of amines 1, 2 and 3 after 72 h in water, freshly-prepared algal growth medium (without algae) and algal growth medium (with algae). | ||
All the experiments evidenced the formation of the parent dibutylamine but to a different extent, depending on the amine used. For all the amines, the hydrolysis is less pronounced in deionized water and poorly affected by the presence of algae; this suggests that the transformation is promoted by chemical species present in the growth medium.
Hydrolysis in the culture medium is more relevant for amine 1 and 3, with a relative abundance of dibutylamine of 47% and 16%, respectively, in comparison to the amount of the tertiary amines in the water phase; amine 2 is much more stable showing less than 5% of hydrolysis, independently by the type of aqueous phase.
For amines 1 and 3 these findings suggest that high levels of dibutylamine formed in the test medium could seriously affect the algal growth since this compound is toxic for algae (the EC50 value of dibutylamine towards Desmodesmus subspicatus is 16 mg L−1).18
According to these results only amine 2 is a good candidate as SHS system; this compound would eventually allow an easy recovery of lipids, while re-using the water for re-growing algae, on the basis of the evidences described: (i) high lipid extraction performances (12.7 wt% and 8.9 wt% with the systems culture/amine volume ratio 1/1 and 2/1, respectively), (ii) low toxicity towards algae (EC50 values 383 mg L−1), and (iii) good stability towards hydrolysis.
Large scale extraction experiments
Once having identified amine 2 as a good candidate, this compound was used to scale-up the system and complete the “switching” cycle (Fig. 7). In fact, in order to provide new results concerning the recovery and reuse of the SHS, and to clearly demonstrate the efficiency of the proposed approach for a full process of sustainable production of third generation biofuels, some relevant process parameters need to be evaluated: recovery of lipids from the SHS by using CO2, amine's contamination of lipid fraction, loss of the amine in the algal growth medium in view of recycling the water phase itself and recycling the amine phase.Fig. 8 shows the results of the extraction process performed on culture volumes 20-fold bigger compared to the small scale (40 mL vs. 2 mL); even in this case 2/1 and 1/1 culture/amine 2 volume ratios were tested.
The kinetics and the amount of biodiesel-like compounds extracted after 24 h were in line with the data obtained on small scale experiments. Analyzing the residual algal pellets after the extraction (Table 3), we found 4.1 wt% and 0.8 wt% of residual fatty acids in the algal pellets with the systems culture/amine 2/1 and 1/1, respectively closing the balance for the total amount of fatty acids contained in N. gaditana. This indicates that, from the point of view of the lipid extraction efficiency, the system 1/1 is able to extract the 95% of TFAs of the alga, whereas the performance of the system 2/1 is around 75%. Having a look to the status of the algal residues, we can see that after the extraction with amine 2, algal cells seemed to remain intact (surrounded by the cell wall, Fig. 9d) even if empty (without chlorophyll, as also suggested by the decreasing in green color of the pellets, Fig. 9b); after 24 h of contact with DMCHA cells were completely broken, suggesting a strongest cellular leakage operated by DMCHA.11
| Culture/amine | TFAs extraction yield (wt%) | TFAs recovery after CO2 addition (wt%) | Residual TFAs (wt%) |
|---|---|---|---|
| a Determined by Karl Fischer titration. | |||
| 2/1 | 10.5 | 9.8 | 4.1 ± 0.6 |
| 1/1 | 13.6 | 12.0 | 0.76 ± 0.01 |
| Culture/amine | H2O in amine phase (wt%)a | Amine in H2O phase (g L−1) | Amine in the extract (wt%) |
|---|---|---|---|
| 2/1 | 1.2 ± 0.1 | 2.0 ± 0.1 | 0.5 |
| 1/1 | 2.2 ± 0.1 | 2.5 ± 0.3 | 0.6 |
 | ||
| Fig. 9 Powders and light microscope images (32×) of N. gaditana before (left) and after (right) 24 h extraction with amine 2. | ||
Besides the lipid extraction capability, the other key parameter related to the utilization of SHS systems in algal cultures relies on the low-energy recovery of the lipid extract by using CO2. Thus the algal water phase was withdrawn, filtered from cellular debris, and re-added to the amine phase (in the case of the system 1/1, 20 mL of algal medium were further added in order to have a double amount of water with respect to the amine volume). After the addition of CO2 for 45 min, and the complete conversion of amine 2 into the water soluble ammonium salt 9, a green layer, containing algal lipids, was collected on the top of the aqueous phase. As indicated in Table 3, the amount of fatty acids in this extract, corresponding to the lipid recovery efficiency, is very high in both cases: 93% with the system 2/1 and 88% with the system 1/1. A lack of 100% recovery could be due to the presence of a certain amount of polar lipids (e.g. shorter chain phospholipids) which could be partially retained in the water phase.
The aqueous phase, containing compound 9, was switched back into amine 2 and water by heating the solution at 70–80 °C for 30 min in order to remove CO2.
The amine layer, which contains 1–2 wt% of water (Table 3), can be re-used for a further cycle of extraction without any other purification.
The water phase, as reported in Table 3, contains around 2 g L−1 of amine: this corresponds to a loss of 0.2% and 0.4% of the amount of the amine initially used for the extraction with the systems 1/1 and 2/1, respectively. Interestingly, a loss of 2 g L−1 is two-times lower than the solubility of amine 2 in pure water (4.5 g L−1): this fact could be explained by a lower solubility due to the salinity of the algal growth medium (35 psu) which has been proven to decreases the solubility of neutral compounds, as for DMCHA.11 Since amine 2 has a potential of inhibiting N. gaditana's growth with an EC50 of 0.3 g L−1 (Table 2), we can assume that the water phase could be reused for re-growing algae after a 10-fold dilution.
Toxicity towards Daphnia magna and ready biodegradability
Dose-response bioassays on aquatic test species such as algae and crustaceans, as well as ready biodegradability determination, are mandatory tools for defining the eco-toxicological profile of chemicals, as required by the EU regulatory framework REACH (regulation (EC) No 1907/2006). Substances hazardous to the aquatic environment are listed in four categories (Category 1 as the most hazardous, Category 4 as the least),20 on the basis of the acute toxicity data (EC50 or LC50 values); if the data indicate that EC50 > 100 mg L−1 then the substance is not classified as hazardous. According to the growth inhibition test on algae (Table 2), amines 1 and 3 can be included in Category 3 (10 mg L−1 < EC50 < 100 mg L−1), whereas amine 2 and DMCHA, for both of which EC50 is above 100 mg L−1, are in the Category 4.Since the impact on aquatic environment should be determined by covering a range of different trophic levels/taxa and/or endpoints, we deepened the eco-toxicological profile of amine 2 by investigating (i) the acute toxicity towards D. magna, and (ii) its biodegradability in water.
The acute toxicity of amine 2 towards the freshwater crustacean D. magna was three-times lower than DMCHA, with an EC50 value of 247 mg L−1 vs. 75 mg L−1 (Table 4); thus, considering this endpoint, amine 2 cannot be classified as hazardous, whereas DMCHA can be included in Category 3.
| Amine | EC50 (mg L−1) |
|---|---|
| 2 | 247 (231–264) |
| DMCHA | 75 (ref. 18) |
Concerning the biodegradability, the OECD guidelines21 report that a compound can be defined as ready biodegradable if its level of biodegradation reaches 60% of ThOD, achieved within 10 days after starting the degradation (the start of the degradation is taken as the time when 10% of the substance has been degraded). As shown in Fig. 10, DMCHA was not biodegradable for the first 7 days of test, after which a rapid increase up to 60% of ThOD within day 17 was achieved. This indicates that bacteria needed to “adapt” to this compound; thus DMCHA, as already reported in the literature, can be considered as readily biodegradable, with a percentage of biodegradation after 28 days of 61%. Opposite to this, amine 2 reached 10% of biodegradation at day 12 but not 60% of ThOD in the following 10 days, so it cannot be classified as promptly biodegradable. The percentage of biodegradation after 28 days was just 34%. The toxicity test, performed by simultaneously testing amine 2 and glucose (as a reference compound), allowed to check if the low biodegradability of amine 2 could be due to the fact that this compound is recalcitrant to biodegradation rather than being inhibitory for the bacteria: this test showed 25% of biodegradation (based on ThOD) occurred within day 10 (so within the day 14). Therefore, according to OECD guidelines, amine 2 is not assumed to be inhibitory for bacteria.
Conclusions
In the present paper we have designed and synthesized new tertiary amines with the aim of widening the range of compounds which can be used as suitable SHS solvents. Our strategy relies on the introduction of polyethoxyalkyl units on a dibutylamine backbone, obtaining compounds which can effectively and reversibly switch from a poor miscibility to a very good miscibility with water. Among the three new synthesized amines, compound 2 resulted to be a valid candidate since it is hydrolytically stable, less volatile and slightly less toxic than DMCHA towards aquatic organisms (algae and crustacean). However it resulted to be less biodegradable than DMCHA. Moreover amine 2 showed similar performances in the extraction of lipids from algal cultures, confirming the opportunity of applicability of SHS in the field of extraction of apolar chemicals from aqueous matrix. Thanks to its low solubility in water, this SHS opens the possibility of recycling the algal culture medium, thus reducing the overall cost and the consumption of water and nutrients in third generation biofuel plants.Experimental
Chemicals
All solvents and chemicals used in this study were obtained from Sigma-Aldrich (purities ≥98%) and were used without purification.Analysis
GC-MS analyses were performed by using a 6850 Agilent HP gas chromatograph connected to a 5975 Agilent HP quadrupole mass spectrometer. The injection port temperature was 280 °C. Analytes were separated by a HP-5 fused-silica capillary column (stationary phase poly[5% diphenyl/95% dimethyl]siloxane, 30 m, 0.25 mm i.d., 0.25 mm film thickness), with helium as carrier gas (at constant pressure, 33 cm s−1 linear velocity at 200 °C). Mass spectra were recorded under electron ionization (70 eV) at a frequency of 1 scan s−1 within the 12–600 m/z range.For all the analysis related to amines 1, 2 and 3 (synthesis, calibration curves, water solubility), the temperature of the column started from 50 °C held for 5 min, then increased up to 325 °C at 10 °C min−1, held for 10 min.
For the analysis of algal lipids, the temperature of the column was increased from 50 °C up to 180 °C at 50 °C min−1, then from 180 °C up to 300 °C at 5 °C min−1. Methyl nonadecanoate was utilized as internal standard for quantification of fatty acid methyl esters (FAMEs). The relative response factors used for the quantitation were obtained by injecting solutions of known amounts of methyl nonadecanoate and commercial FAMEs mixture.
1H and 13C NMR spectra were recorded in CDCl3 and in D2O using a 5 mm probe on a VARIAN Inova 400 spectrometer.
The elemental composition of the three amines was determined by using an elemental analyzer (Thermo Scientific, Flash 2000, Organic Elemental Analyzer) by means of the flash combustion technique.
Synthesis of amines 1, 2 and 3
Amines 1, 2 and 3 were obtained by alkylation of dibutylamine (4) with the corresponding alkyl chlorides (5, 6 and 7) as shown in Fig. 2.2-Chloroethyl methylether (5) was obtained from Sigma-Aldrich (purity 98%) and used without purification.
2-(2-Methoxy-ethoxy)-ethyl chloride (6) was synthesised from the corresponding diethylene glycol monomethyl ether, according to the procedure reported in the literature;22 the crude yellowish product (30 g, >99% yield) was used without further purification. The same procedure was followed to synthesize 2-(2-(2-methoxy-ethoxy)-ethoxy)-ethyl chloride (7) from the corresponding triethylene glycol monomethyl ether (39 g, >99% yield). Compounds 6 and 7 are already known and have been identified by comparison of 1H NMR spectra and GC-MS analysis.
Amine 1 was prepared as follows: dibutylamine 4 (50.7 mmol, 8.5 mL) and K2CO3 (7 g, 50.7 mmol) were charged in a two-neck round bottom flask; 2-chloroethyl methylether (4.8 g, 50.7 mmol) was added and the mixture was stirred at 90 °C for 72 h. The reaction was monitored by GC-MS and stopped when reactant peaks disappeared. After filtering K2CO3 off, amine 1 was purified through an acid–base extraction: the mixture was dissolved in ethyl acetate (20 mL), added to an aqueous solution of HCl (pH 1) and then poured into a separating funnel. After separation of the ethyl acetate, containing traces of 2-chloroethyl methylether, the water layer was treated with aqueous NaOH (pH 10) to regenerate the water-insoluble amine 1 which was extracted three times with ethyl acetate (20 mL). Amine 1 was obtained after evaporation of ethyl acetate under reduced pressure (8.3 g, 44.1 mmol, 87% yield). The same procedure was followed to synthesize amines 2 (10.6 g, 46.1 mmol, 91% yield) and 3 (13.2 g, 48.1 mmol, 95% yield).
The boiling point and the vapor pressure of the three amines were estimated through the EPI (Estimation Programs Interface) Suite™ developed by the EPA's Office of Pollution Prevention Toxics and Syracuse Research Corporation (SRC); the purity was determined by GC-MS spectroscopy, 1H NMR, 13C NMR and elemental analysis (see ESI† for the spectra).
Amine 1, N-butyl-N-(2-methoxyethyl)butan-1-amine (8.3 g, 87% yield).
Found: C, 70.13; H, 13.41; N, 7.69. C11H25NO requires: C, 70.53; H, 13.45; N, 7.48%.
δH (400 MHz, CDCl3) 0.88 (6H, t, J 7.2, (CH3(CH2)3)2N), 1.23–1.29 (4H, m, (CH3CH2(CH2)2)2N), 1.36–1.42 (4H, m, (CH3CH2CH2CH2)2N), 2.42 (4H, dd, J 6.4 and 7.2, (CH3(CH2)2CH2)2N), 2.60 (2H, t, J 6.8, CH3OCH2CH2N), 3.32 (3H, s, CH3O(CH2)2N), 3.42 (2H, t, J 6.4, CH3OCH2CH2N); δC (100 MHz, CDCl3) 14.0 (2C), 20.7 (2C), 29.1 (2C), 53.4, 54.5 (2C), 58.8, 71.2.
GC: rt 13.4 min; m/z (EI) 187 (M+, 10%), 142 (M+-CH3OCH2, 100), 100 (40).
Amine 2, N-butyl-N-(2-(2-methoxyethoxy)ethyl)butan-1-amine (10.6 g, 91% yield).
Found: C, 67.21; H, 12.99; N, 6.04. C13H29NO2 requires: C, 67.48; H, 12.63; N, 6.05%.
δH (400 MHz, CDCl3) 0.87 (6H, t, J 7.2, (CH3(CH2)3)2N), 1.25–1.28 (4H, m, (CH3CH2(CH2)2)2N), 1.37–1.39 (4H, m, (CH3CH2CH2CH2)2N), 2.42 (4H, t, J 7.2, (CH3(CH2)2CH2)2N), 2.64 (2H, t, J 6.8, CH3O(CH2)2OCH2CH2N), 3.36 (3H, s, CH3O(CH2)2O(CH2)2N), 3.50–3.54 (4H, m, CH3O(CH2)2O(CH2)2N), 3.58 (2H, t, J 2.4, CH3O(CH2)2OCH2CH2N; δC (100 MHz, CDCl3) 14.0 (2C), 20.6 (2C), 29.2 (2C), 53.3, 54.6 (2C), 59.0, 69.9, 70.3, 71.9.
GC: rt 17.1 min; m/z (EI) 231 (M+, 10%), 188 (M+-CH3(CH2)2, 30), 142 (M+-CH3O(CH2)2OCH2, 100), 100 (40).
Amine 3, N-butyl-N-(2-(2-(2-methoxyethoxy)ethoxy)ethyl)butan-1-amine (13.2 g, 95% yield).
Found: C, 65.01; H, 12.46; N, 5.07. C15H33NO3 requires: C, 65.41; H, 12.08; N, 5.09%.
δH (400 MHz, CDCl3) 0.85–0.91 (6H, m, (CH3(CH2)3)2N), 1.22–1.28 (4H, m, (CH3CH2(CH2)2)2N), 1.36–1.40 (4H, m, (CH3CH2CH2CH2)2N), 2.41 (4H, t, J 7.2, (CH3(CH2)2CH2)2N), 2.57–2.63 (2H, m, CH3O((CH2)2O)2CH2CH2N), 3.35 (3H, s, CH3O((CH2)2O)2(CH2)2N), 3.49–3.54 (4H, m, CH3O(CH2)2O(CH2)2N), 3.59–3.63 (6H, m, CH3O(CH2)2OCH2CH2N; δC (100 MHz, CDCl3) 14.0 (2C), 20.6 (2C), 29.2 (2C), 53.3, 54.6 (2C), 59.0, 69.8, 70.4, 70.5, 70.6, 71.9.
GC: rt 20.2 min; m/z (EI) 275 (M+, 10%), 232 (M+-CH3(CH2)2, 30), 156 (M+-CH3(O(CH2)2)2O, 10), 142 (M+-CH3(O(CH2)2)2O CH2, 100), 100 (40).
Switching of amines 1, 2 and 3
The “switching” of amines 1, 2 and 3 was performed as follows: each amine (1 mL) was added to D2O (2 mL) at r.t.; then CO2 was bubbled into the mixture for 45 min to obtain a homogeneous phase. This phase was analyzed by 1H NMR and 13C NMR to confirm the “switchability” of the three amines into the corresponding hydrogen carbonates ammonium salts 8–10 (Fig. 4). Each hydrogen carbonate ammonium salts was switched back into tertiary amines and water by stirring at 250 rpm and heating at 70–80 °C for 30 min to remove CO2, until the two phases were fully re-obtained. D2O phase was analyzed again by NMR to confirm the completeness of the conversion (see ESI† for the spectra).Ammonium salt 8, N-butyl-N-(2-methoxyethyl)butan-1-ammonium hydrogen carbonate
δH (200 MHz, D2O) 0.73 (6H, t, J 6.8, (CH3(CH2)3)2N), 1.08–1.27 (4H, m, (CH3CH2(CH2)2)2N), 1.40–1.55 (4H, m, (CH3CH2CH2CH2)2N), 2.97 (4H, t, J 8.4, (CH3(CH2)2CH2)2N), 3.14–3.16 (2H, m, CH3OCH2CH2N), 3.19 (3H, s, CH3O(CH2)2N), 3.55 (2H, t, J 4.4, CH3OCH2CH2N); δC (50 MHz, CDCl3) 12.4 (2C), 18.8 (2C), 24.5 (2C), 51.6, 52.6 (2C), 57.9, 65.3, 160.5.Ammonium salt 9, N-butyl-N-(2-(2-methoxyethoxy)ethyl)butan-1-ammonium hydrogen carbonate
δH (200 MHz, D2O) 0.69 (6H, t, J 3.6, (CH3(CH2)3)2N), 1.10–1.15 (4H, m, (CH3CH2(CH2)2)2N), 1.41–1.44 (4H, m, (CH3CH2CH2CH2)2N), 2.92 (4H, t, J 4.2, (CH3(CH2)2CH2)2N), 3.14–3.16 (2H, m, CH3O(CH2)2OCH2CH2N), 3.13 (3H, s, CH3(O(CH2)2)2N), 3.37–3.39 (2H, m, CH3OCH2CH2O(CH2)2N), 3.43–3.45 (2H, m, CH3OCH2CH2O(CH2)2N), 3.56–3.59 (2H, m, CH3O(CH2)2OCH2CH2N); δC (50 MHz, CDCl3) 12.7 (2C), 19.0 (2C), 24.8 (2C), 52.1, 52.8 (2C), 57.8, 64.0, 69.2, 70.8, 159.9.Ammonium salt 10, N-butyl-N-(2-(2-(2-methoxyethoxy)ethoxy)ethyl)butan-1-ammonium hydrogen carbonate
δH (200 MHz, D2O) 0.69 (6H, t, J 3.8, (CH3(CH2)3)2N), 1.10–1.16 (4H, m, (CH3CH2(CH2)2)2N), 1.39–1.43 (4H, m, (CH3CH2CH2CH2)2N), 2.90–2.94 (4H, m, (CH3(CH2)2CH2)2N), 3.11–3.14 (2H, m, CH3(O(CH2)2)2OCH2CH2N), 3.12 (3H, s, CH3(O(CH2)2)3N), 3.36–3.37 (2H, m, CH3OCH2CH2(O(CH2)2)2N), 3.42–3.45 (6H, m, CH3OCH2CH2O(CH2)2O(CH2)2N), 3.56–3.59 (2H, m, CH3(O(CH2)2)2OCH2CH2N); δC (50 MHz, CDCl3) 12.7 (2C), 19.1 (2C), 24.8 (2C), 52.1, 52.8 (2C), 57.9, 64.0, 69.4 (3C), 70.8, 159.9.Water solubility of amines 1, 2 and 3
The water solubility of amines 1, 2 and 3 was determined by GC-MS trough external calibration. The calibration curve was obtained by analysis of standard solutions prepared as follows: a known amount of amine (about 2 mg) was weighted in a vial; ethyl acetate (2 mL) was added, followed by deionised water (1 mL) and aqueous NaOH (pH 10, 1 mL). The biphasic system was shaken for 30 min and settled for 30 min; the organic phase was withdrawn and calibration standards were firstly obtained by serial dilutions with ethyl acetate and then analyzed.The water solubility of each amine was determined as follows: amine (0.5 mL) was added to deionised water (5 mL) and shaken for 24 h at 500 rpm; the upper amine phase was then removed and aliquots of water (1 mL) were withdrawn, added with aqueous NaOH (pH 10, 1 mL) and ethyl acetate (2 mL), shaken for 30 min and settled for 30 min. The organic phase was withdrawn, diluted with ethyl acetate and analyzed.
The same procedure was used to evaluate the concentrations used in the toxicity tests, and to check the stability of all the amines in all the aqueous media used in the present study.
N. gaditana small scale culture extraction
Nannochloropsis gaditana (Lubián CCMP 527) was obtained from the University of Almeria and grown as previously described.11 The lipid fraction was obtained by extracting dried algal samples (500 mg) with CHCl3/MeOH mixture (45 mL, 2/1 v/v) under reflux for 2 h for three times; after filtration over celite, the combined CHCl3/MeOH layers were evaporated under vacuum. The total fatty acid content (TFAs, sum of bounded fatty acids, BFAs, and free fatty acids, FFAs) was determined as follow: samples (about 2 mg) were dissolved in dimethylcarbonate (0.4 mL). 2,2-dimethoxypropane (0.1 mL) and 0.5 M NaOH in MeOH (0.1 mL) were then added; the samples were placed in an incubator at 90 °C for 30 min. After cooling for 5 min to room temperature, 1.3 M BF3-methanol 10% (w/w) reagent (0.7 mL) was added before repeating the incubation for 30 min. After cooling for 5 min to room temperature, saturated NaCl aqueous solution (2 mL) and hexane (1 mL) containing methyl nonadecanoate (0.02 mg) were added and the samples were centrifuged at 4000 rpm for 1 min. The upper hexane-dimethylcarbonate layer, containing TFAs, was transferred to vials for GC-MS analysis. Each analysis was repeated in duplicate.For the small-scale extraction experiments with SHS, an aliquot (2 mL) of culture (biomass concentration about 2 g L−1) was added to each amine in a round bottom flask (1 mL for the 2/1 culture/amine volume ratio; 2 mL for the 1/1 culture/amine volume ratio). The biphasic mixture was stirred at 500 rpm at room temperature for 24 h (aliquots for analysis were withdrawn after 3 and 6 h) and then centrifuged to recover the upper organic phase. Each extraction was performed in triplicate.
The upper amine layer, containing algal lipids, was used to determine the lipid extraction efficiency of the amine, by converting TFAs into fatty acid methyl esters (FAMEs) by using NaOH/MeOH followed by MeOH BF3, and then analyzing by GC-MS the amount of FAMEs in the extract.
The state of the cells after the extraction was inspected by light microscopy by using an optical microscope (Axiovert S 100, Zeiss) at 32×.
N. gaditana large scale culture extraction
For the large-scale extraction experiments, amine 2 (20 mL, 2/1 culture/amine volume ratio or 40 mL, 1/1 culture/amine volume ratio) was added to the culture (40 mL, biomass concentration about 2 g L−1). The biphasic mixture was stirred at 500 rpm at room temperature for 24 h (aliquots for analysis were hourly withdrawn) and then centrifuged to recover the upper organic phase. The amount of water into the amine phase was determined by Karl Fischer titration; the amount of amine in the water phase was determined by GC-MS as previously described.After determining the lipid extraction efficiency by measuring TFAs content, the amine layer (15 mL in the case of 2/1 culture/amine volume ratio or 30 mL in the case 1/1 culture/amine volume ratio) was used to determine the lipid recovery efficiency upon switching into hydrogen carbonate ammonium salt: algal growth medium (30 mL or 60 mL) without algal cells (they were previously filtered off) was added to the amine and CO2 was bubbled into the mixture for 45 min to obtain a homogeneous phase. During this step, green lipid drops appeared floating on the top of the colorless hydrogen carbonate ammonium salt solution in water, but the amount was not enough to form a separated layer; thus hexane (1 mL) was added to recover the lipid phase. TFAs content in this phase was analyzed as described above. Even the residual lipid content present in the algal pellets after extraction was determined upon conversion into FAMEs following the same procedure. The hydrogen carbonate ammonium salt in the aqueous phase after lipid recovery was then easily reconverted into amine and water by stirring and heating at 70–80 °C for 30 min in order to remove CO2.
Algal growth inhibition tests
Nannochloropsis gaditana (CCMP 1894) was cultured to test the growth inhibition of amines. The toxicity tests were performed according to the ISO 10253: 2006 guideline for testing the growth inhibition of marine microalgae,23 with slight modifications regarding the use of f/2 medium24 and the choice of the test strain, N. gaditana. Experiments were conducted in 50 cm3 sterile Erlenmeyer flasks, sealed with cotton, containing 20 cm3 of sterilized f/2 medium at salinity 35 psu mixed with 5 cm3 of algal suspension in the log growth phase and 5 cm3 of different concentrations of an aqueous amines' solution or f/2 water medium (control cultures). The optical density was measured by using a spectrophotometer (UV/VIS/NIR, JASCO V-650, Tokyo, Japan). The initial OD was 0.1. Three replicates were carried out for each amine concentration tested and for the control. Test concentrations were prepared as follows: f/2 medium (150 mL) was stirred with each amine (5 mL) at 500 rpm for 24 h; after removing the upper amine layer, the f/2 phase was diluted in order to get each desired concentration of amine. The tested concentrations for amine 1 were: 7.3, 14.7, 29.3, 58.6, 117.3, 234.5, 469.0, 938.0, 1407.0 mg L−1; for amine 2 they were: 17.6, 35.2, 70.4, 140.7, 281.4, 562.9, 1125.8, 2251.5, 3377.3 mg L−1; for amine 3 they were: 23.2, 46.4, 92.8, 185.5, 371.1, 742.1, 1484.3, 2968.5, 4452.8 mg L−1.Each flask was placed at 20 °C and illuminated at 90–100 μmol photons m−2 s−1 from daylight type cool white lamps (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 h light–dark period); after 72 h incubation the cell density in the cultures was evaluated by OD. Growth rates were calculated by non-linear regression analyses of the logarithmic of algal growth curves.
8 h light–dark period); after 72 h incubation the cell density in the cultures was evaluated by OD. Growth rates were calculated by non-linear regression analyses of the logarithmic of algal growth curves.
Daphnia magna acute toxicity test
D. magna was cultured in the Centro Interdipartimentale di Ricerca Industriale (CIRI), University of Bologna, (Ravenna, Italy), and maintained at 20 ± 1 °C under a 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 (light
8 (light![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dark) photoperiod. The toxicity of amine 2 towards D. magna was assessed using a 48 h static acute immobilization test according to the procedures set out in the OECD Guideline 202.25 Five neonates (age, <24 h; born from parthenogenic females) were placed each in a 25 mL beaker. Four replicate beakers for each of eight treatment concentrations (control plus seven toxicant concentrations) were prepared and maintained at 20 ± 1 °C under a 16
dark) photoperiod. The toxicity of amine 2 towards D. magna was assessed using a 48 h static acute immobilization test according to the procedures set out in the OECD Guideline 202.25 Five neonates (age, <24 h; born from parthenogenic females) were placed each in a 25 mL beaker. Four replicate beakers for each of eight treatment concentrations (control plus seven toxicant concentrations) were prepared and maintained at 20 ± 1 °C under a 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 (light
8 (light![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dark) photoperiod. Each test vessel was checked for immobilized individuals at 24 and 48 h after the beginning of the test. Animals not able to swim within 15 s after gentle agitation of the test vessel were considered to be immobilized, even though they could still move their antennae. Test concentrations, identified through a preliminary range-finding test, were set in a geometric series ranging from 67.5 to 337.5 mg L−1.
dark) photoperiod. Each test vessel was checked for immobilized individuals at 24 and 48 h after the beginning of the test. Animals not able to swim within 15 s after gentle agitation of the test vessel were considered to be immobilized, even though they could still move their antennae. Test concentrations, identified through a preliminary range-finding test, were set in a geometric series ranging from 67.5 to 337.5 mg L−1.
Biodegradability
Biodegradation was determined by a ready biodegradability test in an aerobic aqueous medium conducted according to the OECD guideline 301F, “Manometric respirometry”.21 The test medium was prepared by adding to distilled water certain concentrations of mineral components from stock solutions (potassium and sodium phosphates plus ammonium chloride, calcium chloride, magnesium sulphate and iron(III) chloride). The bacterial inoculum, derived from an activated sludge taken from a treatment plant receiving domestic sewage located in Ravenna, Italy, was aerated in mineral medium for 5 days at the test temperature.The biodegradability tests were carried out in bottles for 28 days at 20 ± 2 °C. Two different experiments were conducted: in the first one, amine 2 and glucose (reference compound) were tested in triplicate, run in parallel with a blank (containing only inoculum) and a toxicity control (containing amine 2, glucose and inoculum) in duplicate; in the second experiment, amine 2, DMCHA and glucose were tested in duplicate, run in parallel with a blank.
The concentrations of amine 2, DMCHA and glucose were 63, 72 and 100 mg L−1, respectively, corresponding to 161, 208 and 107 mg of Theoretical Oxygen Demand (ThOD) L−1, respectively (ThOD is calculated under the assumption that nitrogen is eliminated as ammonia).
The consumption of oxygen was determined by measuring the change in pressure in the apparatus. Evolved carbon dioxide was absorbed in a solution of potassium hydroxide. The amount of oxygen taken up by the microbial population during biodegradation of the test substance (corrected for uptake by blank inoculum) was expressed as a percentage of ThOD.
Data analysis
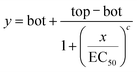
The 50% effect concentration (EC50) of each substance for the algal growth inhibition tests and of amine 2 for the D. magna acute toxicity test was estimated by fitting the experimental concentration–response curves to a logistic model:Where: y = endpoint value (algal growth rate or number of active D. magna); x = substance concentration; bot = expected endpoint value when the concentration of the toxicant is infinite (bottom asymptote); top = expected endpoint value when the concentration of the toxicant is zero (top asymptote); c = slope parameter. The parameters of the equation, including the EC50, were estimated using the non-linear regression procedures implemented in Statistica (Statsoft, Tulsa, OK, USA).
Acknowledgements
We acknowledge the University of Bologna (Ricerca Fondamentale Orientata) and Regione Emilia Romagna (POR-FESR and CIPE) for funding. This study was partly conducted within the framework of the APQ Ricerca Intervento a “Sostegno dello sviluppo dei Laboratori di ricerca nei campi della nautica e dell'energia per il Tecnopolo di Ravenna” “Energia, parte Biomasse” between Università di Bologna and Regione Emilia Romagna (Italy). We thank Dr Cristian Torri of the University of Bologna for Karl Fischer measurements and the support in the analytical procedures.Notes and references
- P. G. Jessop, D. J. Heldebrant, X. Li, C. A. Eckert and C. L. Liotta, Nature, 2005, 436, 1102 CrossRef CAS PubMed
.
- P. G. Jessop, S. M. Mercer and D. J. Heldebrant, Energy Environ. Sci., 2012, 5, 7240 CAS
.
- Y. Liu, P. G. Jessop, M. Cunningham, C. A. Eckert and C. L. Liotta, Science, 2006, 313, 958 CrossRef CAS PubMed
.
- C. I. Fowler, C. M. Muchemu, R. E. Miller, L. Phan, C. O'Neill, P. G. Jessop and M. F. Cunningham, Macromolecules, 2011, 44, 2501 CrossRef CAS
.
- L. Phan, D. Chiu, D. J. Heldebrant, H. Huttenhower, E. John, X. Li, P. Pollet, R. Wang, C. A. Eckert, C. L. Liotta and P. G. Jessop, Ind. Eng. Chem. Res., 2008, 47, 539 CrossRef CAS
.
- R. Hart, P. Pollet, D. J. Hahne, E. John, V. Llopis-Mestre, V. Blasucci, H. Huttenhower, W. Leitner, C. A. Eckert and C. L. Liotta, Tetrahedron, 2010, 66, 1082 CrossRef CAS PubMed
.
- V. Blasucci, C. Dilek, H. Huttenhower, E. John, V. Llopis-Mestre, P. Pollet, C. A. Eckert and C. L. Liotta, Chem. Commun., 2009, 116 RSC
.
- L. Phan, H. Brown, J. White, A. Hodgsonc and P. G. Jessop, Green Chem., 2009, 11, 53 RSC
.
- C. Samorì, C. Torri, G. Samorì, D. Fabbri, P. Galletti, F. Guerrini, R. Pistocchi and E. Tagliavini, Bioresour. Technol., 2010, 101, 3274 CrossRef PubMed
.
- A. R. Boyd, P. Champagne, P. J. McGinn, K. M. MacDougall, J. E. Melanson and P. G. Jessop, Bioresour. Technol., 2012, 118, 628 CrossRef CAS PubMed
.
- C. Samorì, D. López Barreiro, R. Vet, L. Pezzolesi, D. W. F. Brilman, P. Galletti and E. Tagliavini, Green Chem., 2013, 15, 353 RSC
.
- Y. Du, B. Schuur, C. Samori, E. Tagliavini and D. W. F. Brilman, Bioresour. Technol., 2013, 149, 253 CrossRef CAS PubMed
.
- P. G. Jessop, L. Kozycz, Z. Ghoshouni Rahami, D. Schoenmakers, A. R. Boyd, D. Wechsler and A. M. Holland, Green Chem., 2011, 13, 619 RSC
.
- I. Eide-Haugmo, O. G. Brakstad, K. A. Hoff, E. Falck da Silva and H. F. Svendsen, Int. J. Greenhouse Gas Control, 2012, 9, 184 CrossRef CAS PubMed
.
- C. Samorì, P. Galletti, A. Pasteris and E. Tagliavini, Environ. Toxicol. Chem., 2007, 26(11), 2379 CrossRef PubMed
.
- C. Samorì, D. Malferrari, P. Valbonesi, A. Montecavalli, F. Moretti, P. Galletti, G. Sartor, E. Tagliavini, E. Fabbri and A. Pasteris, Ecotoxicol. Environ. Saf., 2010, 73, 1456 CrossRef PubMed
.
- C. Samorì, G. Sciutto, L. Pezzolesi, F. Guerrini, P. Galletti, R. Mazzeo, R. Pistocchi and E. Tagliavini, Chem. Res. Toxicol., 2011, 24, 392 CrossRef PubMed
.
- www.echa.europa.eu.
- C. Samorì, C. Torri, D. Fabbri, G. Falini, C. Faraloni, P. Galletti, S. Spera, E. Tagliavini and G. Torzillo, ChemSusChem, 2012, 5(8), 1501 CrossRef PubMed
.
- www.unece.org.
- Organization for Economic Cooperation and Development Guideline for Testing of Chemicals: Ready Biodegradability, OECD Guideline 301, Paris, France, 1992 Search PubMed
.
- V. Gudipati, D. Curran and C. Wilcox, J. Org. Chem., 2002, 71(9), 3599 CrossRef PubMed
.
- ISO 10253: 2006 Marine algal growth inhibition test with Skeletonema costatum and Phaeodactylum tricornutum.
- R. A. Andersen, Algal Culturing Techniques, Elsevier/Academic Press, San Diego, CA, 2005 Search PubMed
.
- Organization for Economic Cooperation and Development Guideline for Testing of Chemicals: Daphnia Sp, Acute Immobilization Test, OECD Guideline 202, Paris, France, 2004 Search PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: NMR spectra of the synthesised amines and the corresponding ammonium salts. See DOI: 10.1039/c3ra47144f |
| This journal is © The Royal Society of Chemistry 2014 |