A simple sensitive ESIPT on-off fluorescent sensor for selective detection of Al3+ in water†
Junfeng Wanga and
Yi Pang*ab
aDepartment of Chemistry, The University of Akron, Akron, Ohio 44325, USA
bMaurice Morton Institute of Polymer Science, The University of Akron, Akron, Ohio 44325, USA. E-mail: yp5@Uakron.edu
First published on 18th December 2013
Abstract
A highly selective and sensitive fluorescent sensor for Al3+ has been developed. The sensor shows great fluorescence turn-on upon binding Al3+ in complete water, giving strong blue emission. In addition, the sensor's turn-on exhibits excellent selectivity to the Al3+ cation, with only a slight interference from Zn2+. These findings suggest that the developed Al3+ sensor could be a useful molecular probe for practical applications.
Aluminum is the most abundant metal in the Earth's crust and extensively used in modern life.1 The soluble form of aluminum (Al3+), however, is highly toxic to plant growth.2 Excessive aluminum, especially when deposited in the brain even in small amounts, has also been shown to be toxic to humans, and is believed to cause neurodementia such as Parkinson's disease, Alzheimer's disease and dialysis encephalopathy, osteoporosis, etc.3 For these reasons, the development of Al3+ sensors for its facile detection is of great importance in both environmental monitoring and biological assays.
Despite the strong interest, the fluorescent detection for Al3+ cations remains to be a challenging problem. Owing to its weak coordination with ligands and strong hydration ability in water,4 the detection of Al3+ cation is often affected by the existence of interfering metal ions. So far, very few fluorescent chemosensors have been reported for detection of Al3+ with moderate success to date compared to the transition metal ions.5–25 The majority of the reported Al3+ sensors, however, have limitations such as tedious synthetic efforts and/or lack of practical applicability in aqueous solutions.11 Today, almost all the reported dyes for Al3+ have been tested in organic solvents or mixed solvents. In order to enable evaluation of Al3+ ions in aqueous environments, it is highly desirable to develop new sensors, which not only recognize Al3+ ions selectively but also compete effectively with the strong hydration of Al3+ ion during the application in aqueous.
One option is to integrate the Al3+ binding event with the excited state intramolecular proton transfer (ESIPT) in the sensor design. Recently, ESIPT has attracted attention from both theoretical and experimental viewpoints, because it shows a uniquely large Stokes' shifted fluorescence emission (6000–12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1).26 In addition, the ESIPT turn-on or turn-off events will usually lead to a large change in fluorescence wavelength,27 which is of great importance in their practical applications. In general, the ESIPT process requires a proton donor (–OH, –NH2) and a proton acceptor (–C
000 cm−1).26 In addition, the ESIPT turn-on or turn-off events will usually lead to a large change in fluorescence wavelength,27 which is of great importance in their practical applications. In general, the ESIPT process requires a proton donor (–OH, –NH2) and a proton acceptor (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –N
O, –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ) group in close proximity in order to form the intramolecular hydrogen bond (a necessary condition for ESIPT).28
) group in close proximity in order to form the intramolecular hydrogen bond (a necessary condition for ESIPT).28
In order to demonstrate the concept of using ESIPT in Al3+ sensing, we decide to explore the synthesis of Schiff base 1. In the sensor design, the hydroxyl group in 1 forms an intramolecular hydrogen bond with the adjacent imine bond (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), which gives ESIPT. The hydroxyl and adjacent “acetohydrazide” groups also provide a strong binding cavity to host the Al3+ cation. As a consequence, the new sensor integrates the following functions into a single molecule: (a) containing sufficient polar groups to improve water solubility; (b) including an amine group for photoinduced electron transfer (PET) effect to suppress the background signal; and (c) utilizing the Al3+ binding to switch the excited-state intramolecular proton transfer (ESIPT), thereby inducing a large spectral shift. Herein, we report the fluorescence response of sensor 1, which exhibits remarkable fluorescence turned on (by ∼73 fold) upon binding Al3+ ion. In addition, the Al3+ binding also induced a large spectral shift (by 40 nm) (Fig. 1), as the cation binding turned off the ESIPT.
N–), which gives ESIPT. The hydroxyl and adjacent “acetohydrazide” groups also provide a strong binding cavity to host the Al3+ cation. As a consequence, the new sensor integrates the following functions into a single molecule: (a) containing sufficient polar groups to improve water solubility; (b) including an amine group for photoinduced electron transfer (PET) effect to suppress the background signal; and (c) utilizing the Al3+ binding to switch the excited-state intramolecular proton transfer (ESIPT), thereby inducing a large spectral shift. Herein, we report the fluorescence response of sensor 1, which exhibits remarkable fluorescence turned on (by ∼73 fold) upon binding Al3+ ion. In addition, the Al3+ binding also induced a large spectral shift (by 40 nm) (Fig. 1), as the cation binding turned off the ESIPT.
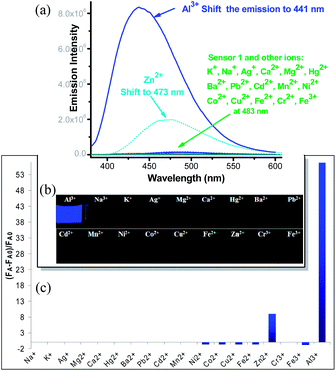
Chemosensor 1 was synthesized in over 90% yield by simple coupling of 2-hydroxybenzaldehyde with acetohydrazide (A) (Scheme 1). Compound 1 could exist in the isomers 1a and 1b, whose ratio was dependent on the equilibrium in different solvents (See ESI Fig. S1–S3†). The structure of the major isomer 1a was determined by X-ray diffraction (ESI Fig. S14†). In aqueous, the free ligand 1 gave very weak green fluorescence (the emission λem = 485 nm, ϕfl = 0.01), partly attributing to the PET effect from the amine. As expected, the emission of 1 exhibited a large Stocks' shift in water (Δλ = 495 (λem) − 317 (λmax) ≈ 168 nm), as a consequence of ESIPT process. Upon addition of Al3+ cation, however, the solution gave bright blue fluorescence, with its quantum efficiency reaching as high as ϕfl = 0.73 (Fig. 1a). In addition, the Al3+ binding also shifted the emission signal (around 40 nm shift from the weak green fluorescence to strong blue fluorescence), which could be used for naked eye detection (See Fig. 1a and b). Clearly, two “oxygen” and one “nitrogen” atoms in sensor 1 provided a strong Al3+ binding (via hard acid–base interaction) to compete with the Al3+ hydration, thereby allowing the reliable fluorescence turn-on in the aqueous solution.
The fluorometric behaviour of 1 was further investigated by addition of the other metal ions Na+, K+, Ag+, Mg2+, Ca2+, Hg2+, Ba2+, Pb2+, Cd2+, Mn2+, Ni2+, Co2+, Cu2+, Fe2+, Zn2+, Cr3+, and Fe3+ in pure water. As shown in Fig. 1a and c, the addition of 5.0 equiv. of Na+, K+, Ag+, Mg2+, Ca2+, Hg2+, Ba2+, Pb2+, Cd2+, Mn2+ and Cr3+ has no obvious effect on the fluorescence emission. The metal ions Ni2+, Co2+, Cu2+, Fe2+ and Fe3+ quenched the fluorescence. Although sensor 1 responded to Zn2+ cation with a slight increase in the fluorescent intensity, the emission wavelength of 1-Zn was only shifted to 473 nm (around 10 nm blue shift). Therefore, sensor 1 is highly selective and sensitive for Al3+ detection, which makes it feasible for biological and environmental applications.
The absorption peak of 1 (λmax = 317 nm) was progressively decreased upon addition of Al3+ (Fig. 2a), which was accompanied with a new band at about 352 nm. The large spectral bathochromic shift indicated the deprotonation, as a consequence of Al3+-binding to phenol. Observation of the distinct isosbestic point at 333 nm suggests the complex formation with one new chemical species. On the basis of Job plot, the complex was assumed to have a ligand-to-metal ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The assumption was supported by high resolution mass spectroscopy (HRMS), which detected m/z 381.1135, corresponding to [2(1-H+)+Al3+]+ (See Fig. 4: the calcd mass for C18H18AlN4O4: 381.1143). Furthermore, mass spectra detected no Al3+ complex with 1
1. The assumption was supported by high resolution mass spectroscopy (HRMS), which detected m/z 381.1135, corresponding to [2(1-H+)+Al3+]+ (See Fig. 4: the calcd mass for C18H18AlN4O4: 381.1143). Furthermore, mass spectra detected no Al3+ complex with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ligand-to-metal ratio from the aqueous solution of 1 and Al3+ (ESI Fig. S5†).
1 ligand-to-metal ratio from the aqueous solution of 1 and Al3+ (ESI Fig. S5†).
The cation binding was further examined by 1H NMR titration. The free ligand exhibited two imine (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) signals at 8.25 and 8.15 ppm, corresponding to the major and minor isomers 1a and 1b respectively (Fig. 3). Upon addition of Al3+ cation, a broad imine signal was developed at 8.45 ppm, attributing to the formation of 1-Al3+ complex. The triplet signal at 7.27 ppm (aromatic Hc) disappeared when ∼0.5 equiv. molar Al3+ was added, indicating that all ligand was consumed to bind Al3+ cation, forming 1-Al3+ complex. Further addition of Al3+ (0.5–1.0 equiv. molar) did not reveal significant change. Since all the ligand was used to bind Al3+, the broad peak at 8.28 ppm was also attributed to 1-Al3+, in addition to the peak at 8.45 ppm. The two peaks at 8.28 and 8.45 ppm suggested possible existence of two different isomers in the metal complex, as the mass spectra detected only 1-Al3+ complex in 2
N–) signals at 8.25 and 8.15 ppm, corresponding to the major and minor isomers 1a and 1b respectively (Fig. 3). Upon addition of Al3+ cation, a broad imine signal was developed at 8.45 ppm, attributing to the formation of 1-Al3+ complex. The triplet signal at 7.27 ppm (aromatic Hc) disappeared when ∼0.5 equiv. molar Al3+ was added, indicating that all ligand was consumed to bind Al3+ cation, forming 1-Al3+ complex. Further addition of Al3+ (0.5–1.0 equiv. molar) did not reveal significant change. Since all the ligand was used to bind Al3+, the broad peak at 8.28 ppm was also attributed to 1-Al3+, in addition to the peak at 8.45 ppm. The two peaks at 8.28 and 8.45 ppm suggested possible existence of two different isomers in the metal complex, as the mass spectra detected only 1-Al3+ complex in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ligand-to-metal ratio.
1 ligand-to-metal ratio.
In order to further evaluate the selectivity of 1 for Al3+ sensing, competition experiments were carried out with other metal cations. The fluorescence response of 1 to Al3+ in pure water in the presence of 5 equiv. of various cations including Na+, K+, Ag+, Mg2+, Ca2+, Hg2+, Ba2+, Pb2+, Cd2+, Mn2+, Ni2+, Co2+, Cu2+, Fe2+, Zn2+, Cr3+ and Fe3+ are given in Fig. 4, respectively. There were insignificant changes in the fluorescence of “1 + Al3+” in the presence of other competing metal cations. Except for Fe3+ and Cu2+, most competing metal ions did not interfere with detection of Al3+ by 1 in H2O (Fig. 4), indicating that 1 can be used as a selective chemosensor for the Al3+ cation.
When using 1 in low concentration (120 nM), the water scattering signal and the background noise could be brought to a observable level (Fig. 5) to evaluate the detection limit. Sensor 1 exhibited a good linear response towards Al3+ in the concentration range of 1.0 to 60 nM in water, showing its analytical value. The detection limit for Al3+ was determined to be as low as 5.0 × 10−11 M (0.5 nM) in pure water (ESI Fig. S15†), which was defined as the three-fold standard deviation of the fluorescence obtained from a blank sample (dye 1 in the absence of Al3+).29,30a The detection limit of 1 for Al3+ cation was about 3 times lower than the highest detection limit reported in DMSO,30b representing a significant advance in sensing Al3+ cation in water.
In an effort to seek the potential biological application, sensor 1 was applied to zebrafish. When zebrafish was exposed to dye 1 and Al3+ in fish tank, bright fluorescence was observed in the fish head and tail (Fig. 6 and ESI Fig. S6†), suggesting that this molecule could be used in organisms at certain conditions.
 | ||
| Fig. 6 Fluorescent images of Al3+ in Zebrafish (a) fish exposed to dye 1 only in the fish tank at the concentration of 10 μM for 2 hours and (b) fish exposed to 10 μM dye 1 and Al3+ for 2 hours. | ||
In conclusion, a highly selective and sensitive fluorescent sensor for Al3+ has been developed. The sensor shows great fluorescence turn-on upon binding Al3+ in aqueous solution, giving strong blue emission. In addition, the sensor's turn-on exhibits excellent selectivity to Al3+ cation, with only a slight turn-on effect observed from Zn2+. These findings suggest that the developed Al3+ sensor could be a useful probe for the application in the biological systems.
Acknowledgements
This work was supported by National Institute of Health (Grant no: 1R15EB014546-01A1). We also thank the Coleman endowment from the University of Akron for partial support, and thank Dr Qin Liu at the University of Akron for assistance in the zebrafish experiment.Notes and references
- (a) G. H. Robinson, Chem. Eng. News, 2003, 81, 54 CrossRef CAS PubMed; (b) The special issue on Aluminium: Lithosphere to Biosphere (and Back), J. Inorg. Biochem., 2005, 99, 1747 CrossRef PubMed.
- E. Delhaize and P. R. Ryan, Plant Physiol., 1995, 107, 315 CAS.
- (a) G. Berthon, Coord. Chem. Rev., 2002, 228, 319 CrossRef CAS; (b) P. Nayak, Environ. Res., 2002, 89, 101 CrossRef CAS; (c) C. S. Cronan, W. J. Walker and P. R. Bloom, Nature, 1986, 324, 140 CrossRef CAS; (d) D. P. Perl, D. C. Gajdusek, R. M. Garruto, R. T. Yanagihara and C. J. Gibbs, Science, 1982, 217, 1053 CAS; (e) D. P. Perl and A. R. Brody, Science, 1980, 208, 297 CAS; (f) D. R. Crapper, S. S. Krishnan and A. J. Dalton, Science, 1973, 180, 511 CAS.
- (a) K. Soroka, R. S. Vithanage, D. A. Phillips, B. Walker and P. K. Dasgupta, Anal. Chem., 1987, 59, 629 CrossRef CAS; (b) T. H. Ma, M. Dong, Y. M. Dong, Y. W. Wang and Y. Peng, Chem. – Eur. J., 2010, 16, 10313 CrossRef CAS PubMed.
- S. Sinha, R. R. Koner, S. Kumar, J. Mattew, P. V. Monisha, I. Kazi and S. Ghosh, RSC Adv., 2013, 3, 345 RSC.
- K. Tiwari, M. Mishra and V. P. Singh, RSC Adv., 2013, 3, 12124 RSC.
- C. H. Chen, D. J. Liao, C. F. Wan and A. T. Wu, Analyst, 2013, 138, 2527 RSC.
- S. Kim, J. Y. Noh, K. Y. Kim, J. H. Kim, H. K. Kang, S. W. Nam, S. H. Kim, S. Park, C. Kim and J. H. Kim, Inorg. Chem., 2012, 51, 3597 CrossRef CAS PubMed.
- Y. W. Liu, C. H. Chen and A. T. Wu, Analyst, 2012, 137, 5201 RSC.
- D. Maity and T. Govindaraju, Chem. Commun., 2012, 48, 1039 RSC.
- Y. Lu, S. Huang, Y. Liu, S. He, L. Zhao and X. Zeng, Org. Lett., 2011, 13, 5274 CrossRef CAS PubMed.
- A. Sahana, A. Banerjee, S. Das, S. Lohar, D. Karak, B. Sarkar, S. K. Mukhopadhyay, A. K. Mukherjee and D. Das, Org. Biomol. Chem., 2011, 9, 5523 CAS.
- S. H. Kim, H. S. Choi, J. Kim, S. J. Lee, D. T. Quang and j. S. Kim, Org. Lett., 2010, 12, 560 CrossRef CAS PubMed.
- D. maity and T. Govindaraju, Chem. Commun., 2010, 46, 4499 RSC.
- K. K. Upadhyay and A. Kumar, Org. Biomol. Chem., 2010, 8, 4892 CAS.
- D. Maity and T. Govindaraju, Inorg. Chem., 2010, 49, 7229 CrossRef CAS PubMed.
- T. H. Ma, M. Dong, Y. M. Dong, Y. W. Wang and Y. Peng, Chem. – Eur. J., 2010, 16, 10313 CrossRef CAS PubMed.
- L. Wang, W. Qin, X. Tang, W. Dou, W. Liu, Q. Teng and X. Yao, Org. Biomol. Chem., 2010, 8, 3751 CAS.
- Y.-W. Wang, M.-X. Yu, Y.-H. Yu, Z.-P. Bai, Z. Shen, F.-Y. Li and X.-Z. You, Tetrahedron Lett., 2009, 50, 6169 CrossRef CAS PubMed.
- W. Lin, L. Yuan and J. Feng, Eur. J. Org. Chem., 2008, 3821 CrossRef CAS.
- A. B. Othman, J. W. Lee, Y. D. Hum, R. Abidi, J. S. Kim and J. Vicens, Tetrahedron, 2007, 63, 10793 CrossRef PubMed.
- Y. Zhao, Z. Lin, H. Liao, C. Duan and Q.-J. Meng, Inorg. Chem. Commun., 2006, 9, 966 CrossRef CAS PubMed.
- S. M. Ng and R. Narayanaswamy, Anal. Bioanal. Chem., 2006, 386, 1235 CrossRef CAS PubMed.
- A. Jeanson and V. Béreau, Inorg. Chem. Commun., 2006, 9, 13 CrossRef CAS PubMed.
- M. Arduini, F. Felluga, F. Mancin, P. Rossi, P. Tecilla, U. Tonellato and N. Valentinuzzi, Chem. Commun., 2003, 1606 RSC.
- J. Goodman and L. E. Brus, J. Am. Chem. Soc., 1978, 100, 7472 CrossRef CAS.
- Y. Q. Xu, Q. Liu, B. Dou, B. Wright, J. Y. Wang and Y. Pang, Adv. Healthcare Mater., 2012, 1, 485 CrossRef CAS PubMed.
- J. E. Kwon and S. Y. Park, Adv. Mater., 2011, 23, 3615 CrossRef CAS PubMed.
- J. D. Ingle and S. R. Crouch, Spectrochemical Analysis; Prentice, 1988, pp. 171–176 Search PubMed.
- (a) K. Tiwari, M. Mishra and V. P. Singh, RSC Adv., 2013, 3, 12124–12132 RSC; (b) K. K. Upadhyay and A. Kumar, Org. Biomol. Chem., 2010, 8, 4892–4897 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: CCDC 970383. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3ra47104g |
| This journal is © The Royal Society of Chemistry 2014 |