Catalytic behavior of supported Ru nanoparticles on the (101) and (001) facets of anatase TiO2†
Fei Wang,
Shitong Zhang,
Changming Li,
Jie Liu,
Shan He,
Yufei Zhao,
Hong Yan,
Min Wei*,
David G. Evans and
Xue Duan
State Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, Beijing 100029, China. E-mail: weimin@mail.buct.edu.cn; Fax: +86-10-64425385; Tel: +86-10-64412131
First published on 7th February 2014
Abstract
Ru/TiO2 heterogeneous catalysts were prepared by immobilizing Ru nanoparticles onto the (101) and (001) facets of anatase TiO2 substrate, and the influence of metal–support interactions on the catalytic behavior of Ru/TiO2 towards CO2 methanation was studied from the viewpoint of electronic structure. Structural investigations based on temperature-programmed reduction (TPR) and X-ray photoelectron spectroscopy (XPS) indicate that a stronger metal–support interaction occurs between Ru and (101) facet in contrast to the Ru and (001) one. This gives rise to an enhancement in CO2 adsorption as well as spill-over hydrogen at the interface of Ru/TiO2(101), accounting for its largely enhanced catalytic activity towards CO2 methanation. In addition, a theoretical study based on density functional theory (DFT) calculations reveals that the Ru nanoparticles supported on the (101) plane have a relatively lower activation energy for CO dissociation (the rate-determining step), which results in their high activity toward CO2 methanation reaction.
1. Introduction
The metal–support interaction has attracted considerable attention in the area of supported heterogeneous catalysis, since the support will have significant influences on the adsorptive capacity,1,2 electron transfer,3,4 and chemical and electronic structure of the active metal.5–7 This will eventually affect the catalytic activity and selectivity. Recently, great efforts have been devoted to the design and fabrication of supported metal catalysts by changing the crystal structure, morphology, or particle size of supports.5,8–11 It has also been reported that specific exposed facets of the support play a key role in determining the metal–support interaction and the resulting catalytic behavior, because the atomic species, coordination environment and electron density of various planes are rather different.5,12 Although several studies have demonstrated that the exposed facet of support nanocrystals could exert profound effect on the catalytic activity and selectivity,13–15 the intrinsic effect of support facet on the metal–support interaction is unclear. A detailed understanding of crystal plane of supports in a heterogeneous catalysis system for the purpose of obtaining largely enhanced catalytic performance remains a challenging goal.In recent decades, global warming from green house gases (mainly CO2) produced by the burning of fossil fuels has attracted increasing attention, and how to achieve the recycle of carbon has become perhaps the most complicated issue. The catalytic hydrogenation of CO2 to give methane, known as methanation, is an efficient approach to recycle exhausted CO2 to give a useful fuel, with potential commercial applications and environmental benefits.16,17 Supported ruthenium (Ru) catalysts hold a prominent position for their extremely high activity in this reaction;18 additionally, the metal–support interaction was also found to impose an essential effect on the catalytic performance. Kowalczyk et al. reported that for Ru catalysts supported on various substrates, the following sequence of TOFs was obtained: Ru/Al2O3 > Ru/MgAl2O4 > Ru/MgO > Ru/C.19 Several studies on this area have also confirmed the catalytic activity is strongly affected by the interaction between Ru nanoparticles and oxide supports both for CO2 methanation and other related reactions.20–22 However, the influence of exposed crystal planes of support on the catalytic behavior of Ru nanoparticles, which is crucial to improve the catalytic efficiency of noble metal from the viewpoint of electronic structure, is not well-resolved.
In this work, active Ru species was respectively supported on two kinds of anatase titanium dioxide (TiO2), i.e., TiO2 nanoparticles (NPs) with exposed (101) plane and nanosheets (NSs) with (001) facet (denoted as Ru/TiO2(101) and Ru/TiO2(001), respectively), and their catalysis evaluation towards CO2 methanation was carried out to shed light on the key role of metal–support interaction. TPR results indicate that a stronger metal–support interaction was found between Ru and (101) facet relative to Ru and (001) one. XPS further shows that there is a stronger electron transfer from Ru nanoparticles to TiO2 (101) facet compared with TiO2 (001) facet, which results in a larger capacity of hydrogen and CO2 adsorption as well as spill-over hydrogen at the metal–support interface of Ru/TiO2(101), accounting for its largely enhanced catalytic activity. DFT calculations further reveal that the Ru species supported on the (101) plane have a relatively lower activation energy for the CO dissociation, leading to its high reactivity toward CO2 methanation. This work provides a detailed understanding of metal–support interaction originating from exposed crystal plane of substrates, which can be used for the design and fabrication of supported heterogeneous catalysts with high performance.
2. Experimental section
2.1 Preparation of TiO2 supports and Ru/TiO2 catalysts
All the reagents were of analytical grade and used without further purification. Anatase TiO2 nanosheets (TiO2-NSs) with exposed (001) facet were prepared by a hydrothermal method similar to that described by Xie et al.23 In a typical experiment, 25 mL of Ti(OC4H9)4 and 3 mL of hydrofluoric acid solution (with a concentration of 50 wt%) were mixed in a 100 mL Teflon-lined autoclave at room temperature, followed by a hydrothermal treatment at 180 °C for 24 h. The resulting white precipitate (TiO2-NSs) was collected, washed with ethanol, distilled water and then 0.1 mol L−1 NaOH solution to eliminate the remaining fluorine, followed by drying at 80 °C for 6 h. Anatase TiO2 nanoparticles (TiO2-NPs) with exposed (101) facet were also prepared by a similar method except that the 3 mL of hydrofluoric acid was replaced by 3 mL of distilled water.The Ru/TiO2 catalysts (Ru/TiO2(101) and Ru/TiO2(001)) were prepared by a deposition–precipitation method. 3.0 g of TiO2 was suspended in 80 mL of water followed by adding 0.1518 g of RuCl3·3H2O (2 wt%) in the suspension. The pH value of the mixture was adjusted to 8.0 by adding 1 mol L−1 Na2CO3 solution. Afterwards, the suspension was stirred for 3 h, and then the solid was filtrated and washed thoroughly, dried in air at 60 °C for 12 h. The product was heated at a constant rate (5 °C min−1) from room temperature to 300 °C in a N2 atmosphere and held for 3 h, followed by a reduction in a H2–N2 mixture (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v) with 100 mL min−1 flow at 300 °C for 3 h.
3, v/v) with 100 mL min−1 flow at 300 °C for 3 h.
2.2 Catalyst characterization
X-ray diffraction (XRD) patterns of samples were obtained on a Shimadzu XRD-6000 diffractometer, using Cu Kα radiation (λ = 0.154 nm) at 40 kV, 30 mA, a scanning rate of 5°/min, a step size of 0.02°/s, and a 2θ angle ranging from 15 to 70°. Elemental analysis of metal in samples was performed using a Shimadzu ICPS-7500 inductively coupled plasma emission spectrometer (ICP-ES). Transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) observations were carried out on a JEOL JEM-2100 transmission electron microscope. Low-temperature N2 adsorption–desorption isotherms of the samples were obtained on a Micromeritics ASAP 2020 sorptometer apparatus. All samples were outgassed prior to analysis at 200 °C for 12 h under 10−4 Pa vacuum. The total specific surface area was evaluated from the multipoint Brunauer–Emmett–Teller (BET) method. X-ray photoelectron spectroscopy (XPS) measurements were performed using an ESCALAB 250 instrument (Thermo Electron) with Al Kα radiation. The binding energy calibration of all spectra was referenced to the C1s signal at 284.6 eV.Temperature programmed reduction (TPR) and temperature programmed desorption (TPD) of the samples were performed by using a Micromeritics ChemiSorb 2720 with a thermal conductivity detector (TCD). Before measurement, the sample (100 mg) placed in a quartz U-tube reactor was degassed under flowing argon at 200 °C for 2 h. For TPR, the sample was reduced in a stream of H2–Ar (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, v/v; 40 mL min−1 total flow) with a heating rate of 10 °C min−1 up to 400 °C. For TPD, the sample (300 mg) was reduced under the same conditions mentioned above, and then cooled to room temperature. Subsequently, TPD measurement was carried out in a stream of argon with a rate of 40 mL min−1 and a temperature ramp of 10 °C min−1.
9, v/v; 40 mL min−1 total flow) with a heating rate of 10 °C min−1 up to 400 °C. For TPD, the sample (300 mg) was reduced under the same conditions mentioned above, and then cooled to room temperature. Subsequently, TPD measurement was carried out in a stream of argon with a rate of 40 mL min−1 and a temperature ramp of 10 °C min−1.
2.3 Evaluation of catalytic performance
The catalytic evaluation of the supported Ru catalysts for CO2 methanation was carried out in a quartz tube reactor (8 mm in diameter) at atmospheric pressure. Brooks mass flow controllers were used to control the gas flow rate. In order to eliminate temperature and concentration gradients, 1.0 g of the catalyst was mixed with 1 mL of inert quartz sand (40 to 60 mesh) and then packed into the reactor. The reactor temperature was controlled by three thermocouples (located near the entrance, at the middle, and near the exit of the bed). After the catalyst pretreatment, the reaction gas mixture consisting of CO2 (15%, v/v), H2 (60%, v/v) and Ar (25%, v/v) at 40 standard cubic centimeters per minute (sccm) total flow rate was introduced into the reactor, and the CO2 conversion was measured over the temperature range 150–350 °C. The product gas stream was analyzed on line by gas chromatography (GC, Shimadzu, 2014C) equipped with a thermal conductivity detector (TCD). The CO2 conversion was calculated based on the CO2, H2 and CH4 mole fractions in the products.2.4 Computational methods
Periodic density functional theory (DFT) calculations were performed using the DMol3 code. The (101) and (001) surface were simulated by a (3 × 3) slab, with a thickness of four titanium layers. According to the previously reported work, the (3 × 3) slab is large enough to reduce the interactions between neighboring images and allows the interfacial strain energy to be fully released. In addition, a vacuum space of 15.0 Å above the surface was employed to eliminate the interaction between two neighboring images along the vertical direction. All the atoms were fully relaxed during the geometric optimization. The generalized gradient approximation with the Perdew–Burke–Ernzerhof functional, together with effective core potentials was utilized. The basis set was specified as the double-numerical basis with polarization functions. The convergence criteria for structure optimizations were based on the following: (1) an energy tolerance of 2.0 × 10−5 Ha per atom; (2) a maximum force tolerance of 4.0 × 10−3 Ha/Å; (3) a maximum displacement tolerance of 4.0 × 10−3 Å. k-space was sampled by the gamma point. The Ru13 cluster was used for simulation in this work, which has been proved to be valid in studying Ru–support interaction previously.24 This cluster size can serve as a useful model for atoms with low coordination number (e.g., those on corners and edges of nanoparticles), which are expected to show high catalytic activity. The adsorption energy (Eads) of species adsorbed on the Ru13/TiO2 surface was calculated from the energy difference between the optimized surface containing the adsorbate (Esurface+adsorbate) and the optimized clean surface with the adsorbate molecule optimized in gas state (Esurface + Eadsorbate), as shown in the following equation:| Eads = Esurface+adsorbate − (Esurface + Eadsorbate) | (1) |
3. Results and discussion
3.1 Structural and morphological study of the catalysts
The morphology of the catalyst supports revealed by TEM and HRTEM is shown in Fig. 1. TiO2-NSs show a typical sheet-like morphology with the lateral particle size of 60–100 nm (Fig. 1A); a well-defined truncated bipyramidal structure was displayed by the HRTEM image (Fig. 1B), with a lattice spacing of ∼0.235 nm parallel to the top and bottom facets. This corresponds to the (001) plane of anatase TiO2, which indicates the top and bottom facets of the nanosheets are (001) planes (Fig. 1C). By comparison, TEM images of TiO2-NPs (Fig. 1D and E) show that the lattice spacing parallel to the side face of the truncated bipyramid is ∼0.35 nm, corresponding to the (101) plane of anatase TiO2. On the basis of the structural information, the percentage of exposed (001) facet for the TiO2-NSs is ∼75%; while the percentage of exposed (101) facet for the TiO2-NPs is large than 90%. | ||
| Fig. 1 (A–C) TEM, HRTEM image and the schematic illustration of TiO2-NSs with exposed (001) facet; (D–F) TEM, HRTEM image and the schematic illustration of TiO2-NPs with exposed (101) facet. | ||
Fig. 2 illustrates the XRD patterns of the two TiO2 substrates and resulting Ru/TiO2 catalysts. All the diffraction peaks match well with the crystal structure of the anatase TiO2 phase (JCPDS no. 21-1272, space group: I41/amd (141)).25 Specially, TiO2-NSs (curve a) exhibit relatively stronger (200) reflection and weaker (004) reflection in comparison with TiO2 NPs (curve b), indicating the predominant exposure of (001) plane. This agrees well with the HRTEM results above and previous reports.26 Moreover, no obvious change in the XRD patterns after loading Ru (curve c and d) was observed, suggesting the maintenance of the structure and morphology of these two TiO2 substrates. It should be noted that Ru species shows no characteristic XRD reflection, probably owing to the high dispersion of Ru NPs with rather small particle size (below the detection limit of XRD).
Fig. 3 shows the TEM and HRTEM images of the two Ru/TiO2 catalysts. It is observed that the original morphology of TiO2 substrate basically remains, and Ru nanoparticles are highly dispersed throughout the support. The histogram of the particle size distribution for Ru/TiO2(001) (Fig. 3C), which is calculated from more than 200 nanoparticles, presents a narrow distribution. The mean particle size was calculated to be ∼1.5 nm. The Ru/TiO2(101) sample shows a similar dispersion of Ru nanoparticles with a mean particle diameter of ∼1.6 nm (Fig. 3D).
 | ||
| Fig. 3 (A and B) TEM images and (C) the histogram of the particle size distribution of Ru/TiO2(001); (D and E) TEM images and (F) the histogram of the particle size distribution of Ru/TiO2(101). | ||
As shown in Table 1, the BET specific surface area decreases slightly from 96 m2 g−1 (TiO2-NSs) to 72 m2 g−1 (Ru/TiO2(001)) with the deposition of Ru nanoparticles; a similar trend was found for the Ru/TiO2(101) sample (decreases from 141 to 115 m2 g−1). This is possibly attributed to partial agglomeration of TiO2 support during the wet impregnation process for the deposition of Ru species. Elemental analysis by ICP-AES reveals the Ru contents are 1.69% and 1.66% in the two catalyst samples (Table 1), which are slightly lower than the nominal content (2%).
| Samples | SBETa/m2 g−1 | Ru loadingb/wt% | TOFCH4c at 150 °C s−1 |
|---|---|---|---|
| a BET surface area.b Determined by ICP-AES.c Turnover frequency of CO2 hydrogenation, which was given as the overall rate of CO2 conversion normalized by the number of active sites over the specified time. | |||
| TiO2-NSs | 96 | — | — |
| TiO2-NPs | 141 | — | — |
| Ru/TiO2(001) | 72 | 1.69 | 2.57 × 10−3 |
| Ru/TiO2(101) | 115 | 1.66 | 4.51 × 10−3 |
3.2 Catalytic activity
The catalytic behavior of the Ru/TiO2(001) and Ru/TiO2(101) samples was evaluated using CO2 methanation as a probe reaction. Fig. 4A shows CO2 conversion vs. reaction temperature over the two catalysts with a reaction-gas feed rate of 40 mL g−1 s−1. For the sample of Ru/TiO2(001), the CO2 conversion increases along with the increase of temperature and reaches to the maximum value of 91.1% at 350 °C. In the case of Ru/TiO2(101) however, the maximum conversion of 98% was obtained at 255 °C, demonstrating an excellent high activity at low temperature. It should be noted that both Ru/TiO2(001) and Ru/TiO2(101) have very satisfactory selectivity towards CH4 (>99%) over the whole temperature range. The turnover frequency (TOF) of the two catalysts was evaluated at low reaction temperature (150 °C), low CO2 conversion (<15%) and high CO2 weight hourly space velocity (WHSV: 360 mL gcat−1 h−1), so as to minimize the effect of transport and water inhibition. The TOF values were calculated to be 4.51 × 10−3 s−1 and 2.57 × 10−3 s−1 for Ru/TiO2(101) and Ru/TiO2(001), indicating a largely enhanced catalytic activity of the former catalyst. The activation energy (Ea) values were also measured by dynamical experiments (shown in Fig. S1†). According to the Arrhenius equation, the Ea of CH4 formation on Ru/TiO2(101) (65.9 kJ mol−1) is lower than that on Ru/TiO2(001) (77.4 kJ mol−1), which agrees with the TOF results.The long-term catalytic stability of the two samples was also investigated. As shown in Fig. 4B, the Ru/TiO2(101) exhibits a stable conversion (95%) at 325 °C for 50 h, with no obvious decrease in its activity (curve a), indicating a sufficient stability for long-term employment. For the Ru/TiO2(001) sample however (curve b), a continuous decrease in the CO2 conversion (from 92% to 87%) at 325 °C was observed with a reaction duration of 50 h. Since the Ru loading and particle size are rather close for these two Ru/TiO2 catalysts, the obvious difference in catalytic performance can be attributed to the support effect originating from the exposed facet, which will be discussed in detail in the next section.
3.3 Influences of metal–support interaction on catalytic performance
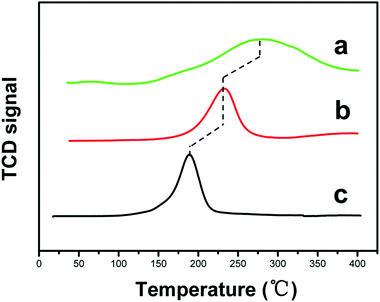
In order to give an insight into the metal–support interaction in these Ru/TiO2 catalysts, TPR was carried out to probe the reducibility of supported Ru catalysts. Fig. 5 shows the TPR profiles of two RuO2/TiO2 catalysts, with pristine RuO2 as a reference sample which was prepared by similar DP method without addition of any support. Only one reduction peak at 188 °C was observed for the pristine RuO2 (curve c), corresponding to the reduction process of Ru4+ to Ru0.27 In the case of the immobilized RuO2, both the RuO2/TiO2(101) and RuO2/TiO2(001) show significantly increased reduction temperature, i.e., at 276 and 233 °C, indicating that TiO2 support hinders the reduction process of RuO2. The RuO2/TiO2(101) sample displays a much higher temperature shift in comparison with RuO2/TiO2(001) one, implying a stronger interaction between Ru species and the (101) facet. Moreover, a larger amount of H2 consumption was found in the RuO2/TiO2(101) catalyst. The values of H/Ru ratio are 23.3 and 11.0 for the RuO2/TiO2(101) and RuO2/TiO2(001), respectively, implying that the TiO2 support in the former system shows a stronger extent of reduction via the spillover mechanism.28Fig. 6 shows the XPS spectra of Ru 3d peak of the two supported Ru catalysts as well as pristine Ru sample. The peak at ∼280.1 for pristine Ru sample (Fig. 6c) can be assigned to Ru 3d5/2, indicative of metallic Ru. Similarly, the peaks of Ru 3d5/2 for Ru/TiO2(101) and Ru/TiO2(001) are observed at ∼280.5 and 280.1 eV, respectively. Interestingly, the binding energy of Ru 3d for Ru/TiO2(101) exhibits a positive shift compared with that of pristine Ru and Ru/TiO2(001), which can be attributed to the modification in the electronic structure of Ru nanoparticles. Such a positive shift indicates an obvious electron transfer from metallic Ru to TiO2 (101) facet.27,29 The positive polarity of Ru species may have influence on the binding energy of the adsorbates and their dissociation process, which will affect the catalytic property.30,31
Hydrogen can be adsorbed in several ways on supported catalysts. TPD has been found to be quite useful for characterizing catalysts by fingerprint spectra and for determining metal surface areas, binding energies and binding states of adsorbed molecules.32 Fig. 7A displays the H2-TPD profiles obtained over the two Ru/TiO2 catalysts. It is observed that hydrogen desorbs from the Ru/TiO2(101) sample (curve a) exhibiting three peaks centered at ∼90, 285 and 480 °C, respectively. According to the results of previous studies,33–35 the low temperature peak (LT, 90 °C) is due to hydrogen chemisorbed at the surface of Ru nanoparticles; the medium temperature peak (MT, 285 °C) is assigned to hydrogen adsorbed at the metal–support interface; the high temperature (HT, 480 °C) hydrogen desorption can be attributed to spilt-over hydrogen or strongly chemisorbed hydrogen. In the case of Ru/TiO2(001) however, its TPD profile is characterized by one main peak located at ∼140 °C (Fig. 7A, curve b), which can be possibly attributed to the hydrogen chemisorbed at the surface of Ru nanoparticles. The absence of MT and HT peak in the Ru/TiO2(001) system excludes the hydrogen adsorbed at the metal–support interface and spillover hydrogen, suggesting a weak synergistic effect between the metallic Ru and (001) facet of TiO2.
 | ||
| Fig. 7 (A) H2-TPD profiles of (a) Ru/TiO2(101), (b) Ru/TiO2(001); (B) CO2-TPD profiles of (a) Ru/TiO2(101), (b) Ru/TiO2(001), (c) TiO2-NPs, (d) Ru/TiO2-NSs, (e) TiO2-NPs-600. | ||
Fig. 7B displays the CO2-TPD profiles of the two TiO2 supports and the resulting two Ru/TiO2 catalysts. TiO2-NPs and TiO2-NSs show rather similar behavior (curve c and curve d), i.e., only one peak occurs at ∼460 °C. In order to confirm the origin of this peak, we performed the CO2-TPD of the TiO2-NPs after a heat treatment at 600 °C for 1 h in Ar atmosphere (denoted as TiO2-NPs-600, Fig. 7B, curve e), in comparison with the TiO2-NPs without heat treatment (Fig. 7B, curve c). The peak at ∼460 °C disappeared for TiO2-NPs-600, which indicates that this peak is not due to the desorption of CO2, but most probably due to the dehydroxylation of the support. The Ru/TiO2(001) sample (curve b) shows almost the same feature with the two supports, indicating that the immobilization of Ru nanoparticles does not contribute to the CO2 adsorption in this system. Interestingly, the Ru/TiO2(101) sample exhibits obviously different character. Besides a shoulder peak (∼460 °C) similar to that of the TiO2-NPs substrate, a strong and broad peak for CO2 desorption was observed at ∼320 °C, which can be attributed to CO2 that adsorbs at the surface of Ru and/or the Ru–TiO2 interface.36 The results demonstrate that a stronger adsorption of reactants (both H and CO2) occurs on the surface of Ru/TiO2(101) catalyst in contrast to the Ru/TiO2(001) one, resulting from the stronger metal–support interaction between Ru and (101) facet of anatase TiO2.
For the methanation of CO2, there is still no consensus on the reaction mechanism. One important proposal involves the conversion of CO2 to CO prior to methanation, followed by the same mechanism as CO methanation, in which the rate-determining step is the formation of surface carbon in CO dissociation.37 To gain a further insight into the catalytic activity of the present catalysts, DFT calculations were carried out to elucidate the structure–activity correlation. In this work, we optimized the structure of Ru13 cluster and subsequently supported them on (101) and (001) surface of TiO2, respectively. This cluster can serve as a suitable model for atoms with low coordination number (e.g., those on corners and edges of nanoparticles), which is expected to show high catalytic activity. The Hirshfeld charge results show that Ru13 is positively charged, indicating the electron transfer from Ru13 to TiO2 (Fig. S2†). The total Hirshfeld charge of Ru13 in Ru13/TiO2(101) is 0.98e, 0.13e higher than that in Ru13/TiO2(001), which is in accordance with the XPS results. In addition, the electronic interaction is mainly localized in the Ru atoms direct contacting with the support; while those in noncontacting layers, are less affected by the electronic interaction. Subsequently, the direct CO dissociation (the rate-determining step) on these two models was studied. Five different adsorption geometries of CO were calculated, and the most stable one was chosen for further calculations. According to the calculated adsorption energies, it is suggested that CO prefers to bind at the Ru–TiO2 interface both in Ru13/TiO2(101) and Ru13/TiO2(001) system (Fig. 8). This adsorption phenomenon has also been observed by Panagiotopoulou previously.38 The adsorption energy of CO on Ru13/TiO2(101) is −1.71 eV, lower than that on Ru13/TiO2(001) by 0.65 eV, indicating that the CO molecule binds more strongly with Ru13/TiO2(101). Moreover, the adsorption energy of CO on the pristine Ru13 cluster is −0.08 eV, much higher than on the supported Ru13 clusters (−1.71 and −1.06 eV, respectively), which indicates that CO binds more strongly on the supported ones. It's known that the energy of CO adsorption is highly sensitive to the electronic state of the adsorption site,39 and the positively charged Ru atoms are desirable for the adsorption of CO. Furthermore, the TPD results above confirm that both H and CO2 adsorb more strongly on the Ru13/TiO2(101) system. It is therefore deduced that the stronger electron transfer from the Ru13 cluster to TiO2 (101) leads to a better stability of chemical species on this catalyst. For this reason, the transition-state (TS) was stabilized considerably for the Ru13/TiO2(101) system, in comparison with the Ru13/TiO2(001) one, rendering a much lower CO dissociation barrier for Ru13/TiO2(101) system (1.25 eV) than that for Ru13/TiO2(001) one (1.37 eV). This accounts for the higher activity of Ru13/TiO2(101) catalyst toward CO2 methanation than that of Ru13/TiO2(001).
4. Conclusions
Ru nanoparticles supported on the (101) facet of anatase TiO2 exhibit significantly higher activity for the catalytic hydrogenation of CO2 to methane than that on the (001) facet. Structural investigations based on TPR and XPS technique give direct evidence that a stronger metal–support interaction occurs between Ru and (101) facet in contrast to Ru and (001) one. This gives rise to an enhancement in reactants adsorption (both H and CO2) at the interface of Ru/TiO2(101), accounting for the observed surprisingly high reactivity and good stability of the Ru/TiO2(101) catalyst. In addition, a theoretical study based on DFT calculations further confirms the stronger electron transfer from Ru cluster to TiO2 (101) facet than to TiO2 (001) one; the Ru species supported on the (101) plane possesses a relatively lower activation energy for the CO dissociation, resulting in its highly catalytic activity toward CO2 methanation reaction. This work provides a fundamental understanding of the metal–support interaction originating from exposed facet of support. It is expected that this strategy can be extended to the design and fabrication of other supported metal catalysts with significantly enhanced behavior in heterogeneous catalysis.Acknowledgements
This work was supported by the 973 Program (Grant no. 2011CBA00504), the National Natural Science Foundation of China (NSFC), the Scientific Fund from Beijing Municipal Commission of Education (20111001002) and the Fundamental Research Funds for the Central Universities (ZD 1303). M. Wei particularly appreciates the financial aid from the China National Funds for Distinguished Young Scientists of the NSFC.Notes and references
- S. J. Tauster, S. C. Fung and R. L. Garten, J. Am. Chem. Soc., 1978, 100, 170–175 CrossRef CAS.
- Z. H. Qin, M. Lewandowski, Y. N. Sun, S. Shaikhutdinov and H. J. Freund, J. Phys. Chem. C, 2008, 112, 10209–10213 CAS.
- X. Y. Liu, M. H. Liu, Y. C. Luo, C. Y. Mou, S. D. Lin, H. K. Cheng, J. M. Chen, J. F. Lee and T. S. Lin, J. Am. Chem. Soc., 2012, 134, 10251–10258 CrossRef CAS PubMed.
- K. Bramhaiah and N. S. John, RSC Adv., 2013, 3, 7765–7773 RSC.
- J. Li, J. L. Chen, W. Song, J. L. Liu and W. J. Shen, Appl. Catal., A, 2008, 334, 321–329 CrossRef CAS PubMed.
- Q. Shen, L. Li, C. He, X. Zhang, Z. Hao and Z. Xu, Asia-Pac. J. Chem. Eng., 2012, 7, 502–509 CrossRef CAS.
- A. S. Ivanova, E. M. Slavinskaya, R. V. Gulyaev, V. I. Zaikovskii, O. A. Stonkus, I. G. Danilova, L. M. Plyasova, I. A. Polukhina and A. I. Boronin, Appl. Catal., B, 2010, 97, 57–71 CrossRef CAS PubMed.
- A. D. Moore, S. M. Holmes and E. P. L. Roberts, RSC Adv., 2012, 2, 1669–1674 RSC.
- C. J. Miller and D. O'Hare, Chem. Commun., 2004, 1710–1711 RSC.
- H. Liu, P. Cheung and E. Iglesia, J. Catal., 2003, 217, 222–232 CAS.
- X. Gao, C. Chen, S. Ren, J. Zhang and D. Su, Chin. J. Catal., 2012, 33, 1069–1074 CrossRef CAS.
- K. B. Zhou and Y. D. Li, Angew. Chem., Int. Ed., 2011, 50, 2–14 CrossRef.
- R. Si and M. F. Stephanopoulos, Angew. Chem., Int. Ed., 2008, 47, 2884–2887 CrossRef CAS PubMed.
- L. J. Liu, Z. J. Yao, Y. Deng, F. Gao, B. Liu and L. Dong, ChemCatChem, 2011, 3, 978–989 CrossRef CAS.
- J. F. Liu, W. Chen, X. W. Liu, K. B. Zhou and Y. D. Li, Nano Res., 2008, 1, 46–55 CrossRef CAS.
- W. Wang, S. P. Wang, X. B. Ma and J. L. Gong, Chem. Soc. Rev., 2011, 40, 3703–3727 RSC.
- J. N. Park and E. W. Mcfarl, J. Catal., 2009, 266, 92–97 CrossRef CAS PubMed.
- P. Panagiotopoulou, D. I. Kondarides and X. E. Verykios, Appl. Catal., A, 2008, 344, 45–54 CrossRef CAS PubMed.
- Z. Kowalczyk, K. Stołecki, W. Raróg-Pilecka, E. Miśvkiewicz, E. Wilczkowska and Z. Karpiński, Appl. Catal., A, 2008, 342, 35–39 CrossRef CAS PubMed.
- E. S. Vasiliadou, E. Heracleous, I. A. Vasalos and A. A. Lemonidou, Appl. Catal., B, 2009, 92, 90–99 CrossRef CAS PubMed.
- L. Wang, J. Chen, L. Ge, V. Rudolph and Z. Zhu, RSC Adv., 2013, 3, 12641–12647 RSC.
- Y. H. Kim and E. D. Park, Appl. Catal., B, 2010, 96, 41–50 CrossRef CAS PubMed.
- X. G. Han, Q. Kuang, M. S. Jin, Z. X. Xie and L. S. Zheng, J. Am. Chem. Soc., 2009, 131, 3152–3153 CrossRef CAS PubMed.
- H. Gao and J. Zhao, J. Chem. Phys., 2010, 132, 234704 CrossRef PubMed.
- Z. Tan, K. Sato, S. Takami, C. Numako, M. Umetsu, K. Soga, M. Nakayama, R. Sasaki, T. Tanaka, C. Ogino, A. Kondo, K. Yamamoto, T. Hashishin and S. Ohara, RSC Adv., 2013, 3, 19268–19271 RSC.
- L. Qi, J. Yu and M. Jaroniec, Phys. Chem. Chem. Phys., 2011, 13, 8915–8923 RSC.
- E. V. Ramos-Fernández, J. Silvestre-Albero, A. Sepúlveda-Escribano and F. Rodríguez-Reinoso, Appl. Catal., A, 2010, 374, 221–227 CrossRef PubMed.
- C. D. Leitenburg, A. Trovarelli and J. Kašpar, J. Catal., 1997, 166, 98–107 CrossRef.
- C. M. Li, S. T. Zhang, B. S. Zhang, D. S. Su, S. He, Y. F. Zhao, J. Liu, F. Wang, M. Wei, D. G. Evans and X. Duan, J. Mater. Chem. A, 2013, 1, 2461–2467 CAS.
- R. L. Martins, M. A. S. Baldanza and M. Schmal, J. Phys. Chem. B, 2001, 105, 10303–10307 CrossRef CAS.
- C. Elmasides, D. I. Kondarides, S. G. Neophytides and X. E. Verykios, J. Catal., 2001, 198, 195–207 CrossRef CAS.
- P. Ferreira-Aparicio, A. Guerrero-Ruiz and I. Rodriguez-Ramos, J. Chem. Soc., Faraday Trans., 1997, 93, 3563–3567 RSC.
- P. Panagiotopoulou and D. I. Kondarides, J. Catal., 2009, 267, 57–66 CrossRef CAS PubMed.
- H. Lin and Y. Chen, Thermochim. Acta, 2004, 419, 283–290 CrossRef CAS PubMed.
- P. Panagiotopoulou and D. I. Kondarides, J. Catal., 2008, 260, 141–149 CrossRef CAS PubMed.
- Y. Zhou, S. Wang, M. Xiao, D. Han, Y. Lu and Y. Meng, RSC Adv., 2012, 2, 6831–6837 RSC.
- S. J. Choe, H. J. Kang, S. J. Kim, S. B. Park, D. H. Park and D. S. Huh, Bull. Korean Chem. Soc., 2005, 26, 1682–1688 CrossRef CAS.
- P. Panagiotopoulou, D. I. Kondarides and X. E. Verykios, J. Phys. Chem. C, 2011, 115, 1220–1230 CAS.
- H. Y. Kim, H. M. Lee and G. Henkelman, J. Am. Chem. Soc., 2012, 134, 1560–1570 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra47076h |
| This journal is © The Royal Society of Chemistry 2014 |