A reticuloendothelial system-stealthy dye–albumin nanocomplex as a highly biocompatible and highly luminescent nanoprobe for targeted in vivo tumor imaging†
Fei-Fei Ana,
Yin-Long Yangc,
Juan Liub,
Jun Yea,
Jin-Feng Zhanga,
Meng-Jiao Zhouc,
Xiu-Juan Zhang*c,
Cai-Jun Zhenga,
Xing-Jie Liangb and
Xiao-Hong Zhang*a
aNano-organic Photoelectronic Laboratory and Key Laboratory of Photochemical Conversion and Optoelectronic Materials, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing 100190, P.R. China. E-mail: xhzhang@mail.ipc.ac.cn
bChinese Academy of Sciences (CAS) Key Laboratory for Biological Effects of Nanomaterials and Nanosafety, National Center for Nanoscience and Technology, No. 11, First North Road, Zhongguancun, Beijing 100190, China
cFunctional Nano & Soft Materials Laboratory (FUNSOM) and Jiangsu Key Laboratory for Carbon-Based Functional Materials & Devices, Soochow University, Suzhou, Jiangsu 215123, P.R. China. E-mail: xjzhang@suda.edu.cn
First published on 19th December 2013
Abstract
A reticuloendothelial system (RES)-stealthy nanoprobe for enhanced tumor imaging is a longstanding pursuit. In this study, a nanocomplex comprising albumin and dye is assembled without crosslinker use. The nanocomplex shows intense luminescence with a photoluminescence quantum yield of up to 0.39 and a large Stokes shift of >130 nm. The nanocomplex also exhibits higher stability against hydrolysis than indocyanine green during the 14 days test. The nanocomplex shows favourable high biocompatibility and can be used for cell labelling. Remarkably, the nanocomplex exhibits six times higher tumor accumulation than that in the liver and spleen. At 96 h post-injection, the nanoprobe is still observable and gives a clear imaging of tumors, which can help in convenient diagnosis post-injection.
1 Introduction
Nanoparticle-based fluorescence nanoprobes are attracting considerable attention because of their high sensitivity and improved tumor accumulation through enhanced permeability and retention (EPR) effects.2,3 However, nanoparticles injected into live bodies are generally recognized as foreign particles and cleared by phagocytes, resulting in high uptake by the reticuloendothelial system (RES), mainly including the liver and spleen.4,5 This high accumulation hampers their delivery to desired tumor sites and potentially induces undesired organ toxicity. To create stealthy nanoparticles, the nanoparticles surfaces could be coated with polyethylene glycol (PEG), zwitterionic ligands, etc.6–10 Recently, a peptide that can successfully cheat phagocyte recognition and inhibit RES clearance was synthesized to enhance the delivery of foreign nanoparticles to a tumor.1,5 However, the surface-modified nanoparticles still show higher accumulation or stronger background signal in the liver than in a tumor, leading to unsatisfactory diagnosis effects.1,11 These strategies are efforts on improving the stealth property of foreign particles. Alternatively, we can use endogenously and inherently RES-stealthy materials as carriers for delivering imaging agents to tumor.Albumin is synthesized in the liver with less opsonization by RES through an aqueous steric barrier.12,13 Albumin has been also used as active targeted carriers for drugs and contrast agents but requires sophisticated chemical conjugation with targeted ligands. Accordingly, an extra step involving catalyst use and removal is needed.14–19 To improve passive tumor accumulation caused by EPR effects, glutaraldehyde was used as crosslinker to prepare nanosized albumin particles in previous studies.20 However, residual free glutaraldehyde is toxic to cells because it can crosslink vital cell proteins and should thus be carefully removed.21,22 The uncertain cytotoxicity of crosslinker would be an obstacle to the clinical approval of albumin nanoparticle related biomaterials. Thus, the development of crosslinker-free protein nanoparticles would benefit the clinical application of albumin-based nanomaterials. Furthermore, previously reported albumin nanoparticles for non-invasive tumor imaging possess a relatively moderate photoluminescence quantum yield (PLQY; 0.12).20 Therefore, highly emissive albumin nanoparticles prepared without using a crosslinker and able to maintain RES stealth are desirable for tumor-targeted imaging.
In this study, we present a RES-stealthy nanoprobe with high biocompatibility and high PLQY constructed by assembling a dye and albumin (Scheme 1). The nanoprobe is a supramolecular nanocomplex without any crosslinker added and has the ability to maintain a stable diameter distribution in water and saline. The nanocomplex preserves robust stability against hydrolysis for more than 14 days and exhibits a remarkably higher PLQY (0.39) than indocyanine green (ICG; 0.027 ± 0.005) ascribed to the rigid molecular structure of the dye. In vitro studies demonstrate that the nanoprobe can be utilized for cell labeling with high biocompatibility. Noninvasive in vivo imaging shows the suitability of the nanocomplex as a tumor-targeted fluorescence nanoprobe.2,3 Ex vivo organ biodistribution analysis indicates six times higher tumor accumulation than in the liver and spleen, which can reduce potential side effects in these organs. More importantly, long-term tumor imaging of the resulting nanocomplex is observed for over 4 days, which enables investigations over a significant period of time. Those investigations may include cancer cell migration and metastasis tracking. Therefore, this study provides a useful strategy for designing highly RES-stealthy, biocompatible, luminescent, and tumor-targetable bioprobes by combining albumin with dyes having a rigid molecular structure.
2 Experimental
2.1 Materials and characterization
Bovine serum albumin was purchased from Suzhou Alpha Biotech Co., Ltd. 2,7-Di(4-(diphenylamino)phenyl-2,1,3-benzothiadiazol-7-yl)-9,9′-spirobifluorene (Spiro-BTA) was synthesized according to literature.23Dynamic Light Scattering (DLS) was used in characterizing nanocomplex sizes in the aqueous solution (Malvern, UK). TEM image was obtained through a FEI Tecnai G2 F20 S-Twin TEM (Hillsboro, OR). Emission spectra of the nanocomplex were characterized using a fluorometer (FluoroMax 4, Horiba Jobin Yvon, Edison, NJ). UV-Vis-NIR spectra were obtained using a LAMBDA 750 UV/Vis/NIR spectrophotometer (Perkin Elmer). Finally, in vitro cell imaging was characterized with a confocal laser microscope (Leica, TCS-SP5).
2.2 Preparation of hydrophobic dye–albumin nanocomplex and dye loaded DSPE-PEG nanomicelle
First, 250 mg bovine serum albumin was dissolved in 50 mL deionized water through magnetic stirring. Consequently, 6 mL THF solution of Spiro-BTA (0.33 mg mL−1) was added dropwisely under magnetic stirring. After 30 min, THF in the solution was removed via rotary evaporation. The remaining solution was centrifuged at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 rpm for 10 min. The supernatant was collected and further concentrated through ultrafiltration. Finally, the sample obtained was stored at 4 °C for use. The ratio of the dye encapsulated inside the albumin is estimated to be 0.8% determined by UV-Vis-NIR spectra.
800 rpm for 10 min. The supernatant was collected and further concentrated through ultrafiltration. Finally, the sample obtained was stored at 4 °C for use. The ratio of the dye encapsulated inside the albumin is estimated to be 0.8% determined by UV-Vis-NIR spectra.
Spiro-BTA loaded nanomicelles (M-Dye) were prepared from DSPE-PEG2000 and Spiro-BTA via the film dispersion method. Spiro-BTA and DSPE-PEG2000 were dissolved in chloroform at room temperature at a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50. The solvent was removed by vacuum rotary evaporation to form a dry dye-containing lipid film. The dried film was hydrated with phosphate-buffered saline (PBS; pH 7.4) at 60 °C for 30 min. Non-encapsulated Spiro-BTA was separated by filtration of the nanomicelle suspension through a 200 nm polycarbonate membrane (Millipore Co., Bedford, MA).
50. The solvent was removed by vacuum rotary evaporation to form a dry dye-containing lipid film. The dried film was hydrated with phosphate-buffered saline (PBS; pH 7.4) at 60 °C for 30 min. Non-encapsulated Spiro-BTA was separated by filtration of the nanomicelle suspension through a 200 nm polycarbonate membrane (Millipore Co., Bedford, MA).
2.3 Photophysical properties
PLQY of Spiro-BTA nanocomplex was measured with an integrating sphere attached to an Edinburgh Instruments (Livingston, UK) FLS920 fluorimeter. The UV-Vis-NIR spectra were obtained with a LAMBDA 750 UV/Vis/NIR spectrophotometer (Perkin Elmer). Emission spectra of the solutions were characterized with a fluorometer (FluoroMax 4, Horiba Jobin Yvon, Edison, NJ).The in vitro fluorescence imaging was characterized using Maestro in vivo fluorescence imaging system (CRi Inc.). Green light with a central wavelength at 523 nm was used as the excitation source; 1 μg mL−1 Spiro-BTA nanocomplex in centrifuge tube was scanned and recorded at the wavelength region of 560–720 nm with 200 ms exposure time.
2.4 In vitro stability of nanocomplex
The Spiro-BTA nanocomplex was dispersed in deionized water and saline respectively. Afterwards, the diameters of the nanocomplex were measured with DLS at each set time. The statistical results of the diameter distribution were presented by number.The stability against hydrolysis was characterized by the change of optical density (OD). The Spiro-BTA nanocomplex and indocyanine green (ICG) solutions with OD ∼ 1 at the starting day were stored in centrifuge tube without special protection. Their OD were measured every other day.
2.5 Nanocomplex for in vitro cell imaging
Pretreated glass coverslips were placed at the bottom of the 24-well plates in advance. Afterwards, 0.75 mL complete medium with human mouth epidermal carcinoma (KB) cells were seeded in 24-well plates. After 24 h, each well was added with 0.05 mL sterilized Spiro-BTA nanocomplex solution. After 6 h of further incubation, the cells in each well were rinsed with PBS buffer three times. The cells in each well were then fixed with 4% paraformaldehyde (water solution) for 30 min. Finally, the cells on coverslips were sealed, to be used in later imaging with a confocal laser microscope.2.6 Cytotoxicity assessment of Spiro-BTA nanocomplex
0.1 mL complete medium with KB cells were seeded in 96-well plate (6000 cells per well) and incubated for 24 h. Afterwards, a 0.025 mL sterilized Spiro-BTA nanocomplex solution with varying concentration levels of PBS buffer was added to each well for incubation (37 °C, 5% CO2). After 24 h, the medium was removed and each well was washed with PBS buffer twice. A solution of 0.02 mL thiazolyl blue tetrazolium bromide (5 mg mL−1 in deionized water) was added to each well. After 4 h, the solutions were subsequently removed and washed with PBS buffer twice. Then, 0.2 mL DMSO was added, and the plate was shaken for another 10 min. Accordingly, the relative viable cells were finally quantified using a spectrophotometer by measuring the absorbance at 570 nm (ELISA reader). The cell viabilities of the wells without Spiro-BTA nanocomplex treatment were set to be 1.0, while others were calculated as relative values.2.7 Blood circulation time of nanocomplex
Blood circulation was measured by drawing ∼10 μL of blood from the tail vein of KB tumor bearing Bal b/c mice post injection of Spiro-BTA nanocomplex. Thereafter, each blood sample was dissolved in 1 mL of lysis buffer (1% SDS, 1% Triton X-100, 40 mM Tris–acetate). The concentration of Spiro-BTA nanocomplex in the blood was determined by the fluorescence spectrum of each solubilized blood sample using a FluoroMax 4 fluorometer (HORIBA Jobin Yvon, France). To obtain a standard calibration curve, a series of dilutions of the Spiro-BTA nanocomplex solution were measured. A blank blood sample, that is, without Spiro-BTA nanocomplex injection, was measured to determine the level of blood auto-fluorescence, which was subtracted from the fluorescence intensities of injected samples during calculation of the concentration.2.8 Xenograft tumor models
KB cells were cultured in the standard media as recommended by the American type culture collection (ATCC). Female Bal b/c athymic nude mice experiments were obtained from Suzhou Belda Bio-Pharmaceutical Co., Ltd. and performed under protocols approved by the Soochow University Laboratory Animal Center. The KB tumor models were generated by the subcutaneous injection of 5 × 106 cells in ∼100 μL serum-free RMPI-1640 medium onto Bal b/c nude mice. The mice were injected with Spiro-BTA nanocomplex after 10 days.2.9 In vivo biodistribution comparison of nanocomplex and DSPE-PEG nanomicelle
Three mice bearing the KB tumor were intravenously injected with 200 μL solution of 0.9 mg mL−1 Spiro-BTA nanocomplex and imaged using the Maestro in vivo fluorescence imaging system (CRi Inc.). After 24 h, the mice were sacrificed and dissected, and the organs of each mouse were collected and imaged. Green light, with a central wavelength at 523 nm, was used as the excitation source. Spectral imaging from 560 nm to 720 nm (10 nm step) was performed, with an exposure time of 200 ms for each image frame. Auto-fluorescence was removed using the spectral unmixing software. The average fluorescence intensity of each organ was calculated using software. The average fluorescence intensity of each organ was calculated as the relative value of the tumor. The biodistribution of Spiro-BTA loaded DSPE-PEG nanomicelles was studied and calculated using the same procedures and methods described above.2.10 In vivo tumor imaging by nanocomplex
The tumor-bearing mice were intravenously injected with 200 μL solution of 0.9 mg mL−1 Spiro-BTA nanocomplex and imaged using the Maestro in vivo fluorescence imaging system (CRi Inc.). Green light, with a central wavelength at 523 nm, was used as the excitation source. In vivo spectral imaging from 560 nm to 720 nm (10 nm step) was performed, with an exposure time of 200 ms for each image frame. Auto-fluorescence was subsequently removed using the spectral unmixing software.For prolonged in vivo tumor imaging, mice were continuously imaged with the Maestro in vivo fluorescence imaging system at 24 h, 48 h, 72 h, and 96 h post intravenous injection. The obtained images subtracted auto-fluorescence by using a spectral unmixing software.
3 Results and discussion
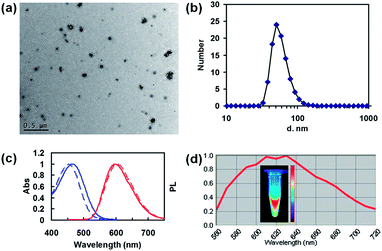
The nanocomplex was obtained by precipitating albumin from deionized water via adding the THF solution (Scheme 1). The albumin dissolves perfectly in water, but is poorly soluble in THF. Upon the addition of the Spiro-BTA solution in THF, the albumin solubility in the aqueous solution gradually decreased, and finally aggregated into a nanocomplex. The diameter of most nanocomplex is confirmed to be 30–110 nm by TEM (Fig. 1a). DLS analyse shows a diameter of ∼51 nm with polydispersity of 0.219 (Fig. 1b). As a hydrophobic dye, Spiro-BTA molecules aggregate and entangle with the hydrophobic domains of the albumin chains once added into the aqueous solution. Thus, the Spiro-BTA is trapped inside the nanocomplex during the formation of the nanocomplex. The zeta potential of the as-prepared nanocomplex was −3.98 mV in deionized water and −2.62 mV in saline water, suggesting that the outer electric double layers are significant in the process of stabilizing the nanocomplex in aqueous solutions.Fig. 1c shows the absorption and emission spectra of nanocomplex with 0.8% Spiro-BTA loading in the aqueous solution, and pure Spiro-BTA in the tetrahydrofuran (THF) solution. The absorption and emission peaks of nanocomplex embedded with Spiro-BTA are similar to those observed in THF solution. The emission of the Spiro-BTA embedded nanocomplex at 598 nm was slightly blue-shifted (4 nm) compared to that of the Spiro-BTA in THF. Significantly, the Stokes shift of the nanocomplex is as large as 130 nm, whereas most organic dyes share with small Stokes shift. The large Stokes shift of the nanocomplex is advantageous in improving the detection sensitivity by avoiding the interference between excitation and emission light. The PLQY of the nanocomplex was determined as 0.39 by an integrating sphere which is much higher than that of the ICG (0.027 ± 0.005) in a water solution. This can be attributed to the excellent photophysical property of Spiro-BTA. It is reported that Spiro-BTA possessed rigid molecular structure and exhibited a PLQY of 0.51 in solid film, which is almost the same as that in dilute solution, showing no characteristics of aggregation-induced fluorescence quenching.23 To confirm the feasibility of using the nanocomplex for in vivo bioimaging, the fluorescence property was characterized by Maestro in vivo fluorescence imaging system (CRi Inc.). Under green light excitation (200 ms exposure time), the aqueous solution of the nanocomplex (1 μg mL−1 of Spiro-BTA) in the centrifuge tube shows that strong emission at the wavelength region of 560–720 nm is sensitively recorded by the camera of the imaging system (Fig. 1d). The nanocomplex emission spectra obtained by Maestro in vivo fluorescence imaging system was slightly different from that by fluorometer. This difference can be attributed to the interferences of the background noise and the sensitivity difference of the instruments in the spectra region.
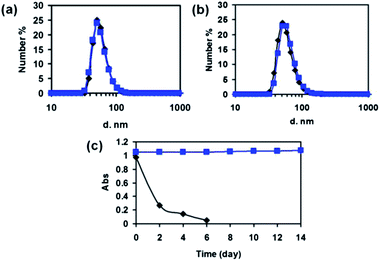
To avoid the potential cytotoxicity, cross-linker was not used during the preparation of the nanocomplex. Instead, it was prepared as a supramolecular nanocomplex of albumin and Spiro-BTA dye. The diameter stability of the nanocomplex is affirmed by measuring the diameter variation overtime with DLS. On the 46th day, the diameter distribution of the nanocomplex is almost unchanged comparing with the starting day in deionized water (Fig. 2a). To further confirm the diameter stability, the nanocomplex was dispersed in saline and measured with DLS. As shown in Fig. 2b, the diameter of the nanocomplex slightly increased from 50.75 nm to 58.77 nm in 48 h. The relatively high diameter stability of the nanocomplex is critical for the successful in vivo targeted tumor imaging. Nanoparticles that have a diameter smaller than 200 nm are known to have the ability to accumulate efficiently at the tumor by EPR effect.2,3 This diameter stability is assumed to be resulted from the slow dissociation of aggregated albumin generated upon the addition of THF solution.
Since many dyes, such as ICG, suffer from serious hydrolysis which limits their practical application, the resistance to hydrolysis of the as-designed nanocomplex is determined by the absorption change. For comparison, the water solution of the ICG and the nanocomplex were both placed in laboratory room without any light shedding or cold storing. The absorption of ICG decreased quickly to less than 30% in two days, and was almost undetectable for 6 days; on the other hand, the absorption of the nanocomplex maintained almostly unchanged in a prolonged period of 14 days (Fig. 2c). The characterization results by absorption change suggests that the nanocomplex is sufficiently resistant to hydrolysis comparing with ICG dyes. The high stability of nanocomplex in aqueous solution is highly advantageous for optical imaging, especially for long-term bioimaging and tracking.
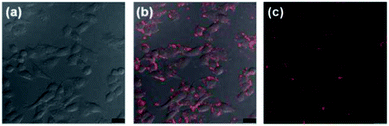
KB tumor is known to be typical tumor near the skin which could be diagnosed by fluorescence imaging. In addition, widely recognized EPR in KB tumor facilitates the efficient fluorescence diagnosis in vivo with nanoprobes by passive accumulation. Thus, the as-prepared nanocomplex was further explored as nanoprobes for KB cells labeling in vitro and tumor imaging in vivo. Having high PLQY and resistance to hydrolysis, the nanocomplex was firstly utilized for in vitro optical bioimaging of KB cells. The KB cells were subsequently treated with Spiro-BTA nanocomplex (5 μM) for 6 h, and then imaged with confocal laser scanning microscopy (Fig. 3). The bright-field images showed that the morphologies of all the KB cells (treated with nanocomplex) did not change, and they were viable after the sample treatment. This result indicated that the Spiro-BTA nanocomplex did not induce obvious toxicity to the cells. The fluorescence images demonstrated that the fluorescence of nanocomplex could be clearly observed from the periphery of the treated KB cells, indicating that the KB cells were effectively labeled by the nanocomplex. The effective fluorescence labeling of KB cells with the nanocomplex might be utilized for tracking the cancer cell migration in vitro and perhaps metastasis in vivo.
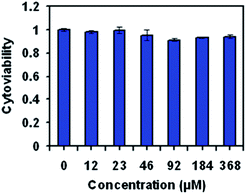
The biocompatibility of nanoparticles is critical to their application as nanoprobes for biolabeling and bioimaging because higher cytotoxicity might interfere the normal activity of the labeled cells and result in false results in certain applications, such as failed cell migration and metastasis tracking. The in vitro cytotoxicity of the Spiro-BTA nanocomplex against KB cancer cells was studied using standard 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cell-viability assay. After incubating with the as-prepared nanocomplex for 24 h, the KB cells maintained cell viability of greater than 90% at a high concentration of 368 μM (calculated by the concentration of Spiro-BTA dyes) (Fig. 4). The high cell viability of KB cells within the tested period of time suggests that the nanocomplex had low cytotoxicity and good biocompatibility. The low cytotoxicity is attributed to the absence of heavy metal ions in the nanocomplex, which makes the nanocomplex suitable for fluorescence bioimaging applications.
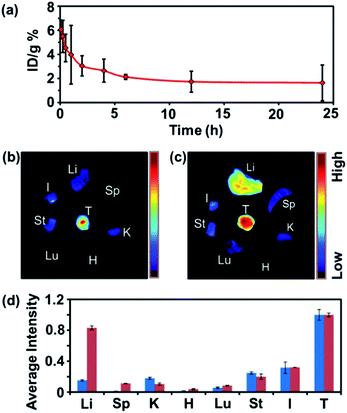
The Spiro-BTA nanocomplex concentrations in the blood over time were measured after the intravenous injection of Spiro-BTA nanocomplex into the Bal b/c mice. At each set time post injection, blood (∼10 μL) was drawn and solubilized using a lysis buffer. The fluorescence of lysized blood was measured with a fluorometer to determine the nanocomplex concentration. A blood circulation half-life of ∼2 h was observed for the Spiro-BTA nanocomplex, which is considerably longer than that of ICG (2–4 min) and comparable with some other nanoparticles for effective tumor targeting in vivo, such as PEGylated nanographene (Fig. 5a).24,25 The longer blood circulation time could be advantageous in improving the accumulation of the nanocomplex at the tumor site as compared to ICG.
To investigate the biodistribution of the Spiro-BTA nanocomplex, the KB bearing Bal b/c mice were sacrificed at 24 h post injection. Various organs were collected and spectrally imaged using the Maestro system (Fig. 5b). The tumor exhibited the strongest fluorescent signal, which indicated the high accumulation of nanocomplex at the tumor. Interestingly, fluorescent signals of the nanocomplex in the RES, mainly including liver and spleen, were rather lower than that of the tumor. The exceptionally high tumor and low RES accumulation indicate that the as-prepared nanocomplex is an excellent nano-vehicle for specifically delivering poorly water-soluble molecules to the tumor without obvious liver and spleen accumulation. Spiro-BTA loaded DSPE-PEG nanomicelle was also prepared and intravenously injected into the mice with the same dosage. 24 h later post injection, various organs were collected and spectrally imaged using the Maestro system (Fig. 5c). The tumor exhibited the strongest fluorescent signal, however, the signals in the liver and spleen were also strong and clearly seen which indicated a relatively high accumulation of Spiro-BTA loaded DSPE-PEG nanomicelle at RES organs.
To further compare the biodistribution of Spiro-BTA nanocomplex and Spiro-BTA loaded DSPE-PEG nanomicelle in vivo, the signal of each imaged organ area (with subtracted background) was collected and calculated as average fluorescence intensity which semiquantitatively gives the biodistribution analysis results of both nanoprobes in vivo (Fig. 5d). The fluorescence intensity of each organ was calculated as the relative value of respective tumor. The average fluorescence intensity of each organ shows that there is only 15% nanocomplex locates in the liver relative to the tumor, while 83% DSPE-PEG nanoparticle locates in the liver relative to the tumor. In addition, there is only 0.8% nanocomplex locates in the spleen relative to the tumor, while 11% DSPE-PEG nanoparticle locates in the spleen relative to the tumor. The comparison results of biodistributions demonstrate the obvious RES-stealthy advantage of our nanocomplex over traditional DSPE-PEG nanoparticles. The good RES-stealthy property of the nanocomplex can be ascribed to the inherent RES-stealthy property of albumin, which has a half-life of 19 days in blood circulation.11 Though the blood circulation time of the nanocomplex is known to be only 2 h (Fig. 5a), which is shorter than that of the free albumin in blood, the hydrodynamic diameter (around 50 nm in Fig. 1b) of the nanocomplex benefits its accumulation at tumor by EPR effects.2,3 The as-prepared nanocomplex is the noncovalent supramolecular nanocomplex of albumin without crosslinking, which maintains the highest level of the albumin's RES-stealth. The good RES-stealthy property of the nanocomplex could be utilized for chemotherapeutic agents delivery without much liver and spleen toxicity.
To verify the viability of the application of Spiro-BTA nanocomplex for in vivo tumor targeting, the 200 μL saline solution of the nanocomplex was intravenously injected into nude mice bearing subcutaneous KB tumor xenografts. The KB bearing Bal b/c mice were imaged at 24 h post injection, with Spiro-BTA nanocomplex and imaged with the Maestro system. The fluorescence signal at the tumor site was obtained by automatically unmixing the spectral signatures of the whole mice body scan (Fig. 6a and b). The fluorescence signal at the tumor site was significantly stronger than any other part of the mice body, indicating that the nanocomplex as good fluorescence probe for effective tumor diagnosis. The spectral signatures from the tumor sites and the auto-fluorescence of the skin sites were respectively presented in Fig. 6c. The fluorescence spectra of signals were noticeably consistent with the fluorescence spectra region of the Spiro-BTA nanocomplex. Moreover, it could easily be differentiated from the auto-fluorescence at skin sites. The excellent tumor-imaging result was attributed to the high passive accumulation at tumor site, which resulted in high signals at the tumor and low background in other tissues and organs. Though the emission peak of the nanocomplex is around 600 nm and not located in the near-infrared (NIR, 700–900 nm) window, the high signal-to-noise ratio was useful for unmixing the spectral signatures of the whole mice body scan and provided a clear image of tumor site. The experimentation results have suggested that the Spiro-BTA nanocomplex can be utilized as bright nanoprobes for potential applications in in vivo tumor targeting and diagnoses.
The nanocomplex has been demonstrated to be considerably more stable than ICG against hydrolysis in two weeks (Fig. 3), and is thus suitable for long-term in vivo imaging. Fig. 7 shows fluorescence images of a KB bearing mouse obtained at the time points of 24, 48, 72, and 96 h after intravenous injection of the nanocomplex with a dose of 9 mg Spiro-BTA per kg. The tumor has been imaged by a Maestro optical imaging system throughout the timeframe of the experiment period. The fluorescence signal at the tumor was retained long enough for detection at 96 h postinjection, which might be used for monitoring the expansion of tumor growth in vivo. These results imply that the fluorescence of the Spiro-BTA nanocomplex is not quenched and remains stable in vivo and is slowly cleared or metabolized in vivo. Therefore, the highly tumor accumulative Spiro-BTA nanocomplex can be utilized not only for tumor diagnoses at any given point in time, but also for further investigations over a significant period of time. Those investigations may include cancer cell migration and metastasis tracking.
 | ||
| Fig. 7 In vivo fluorescence images of KB tumor-bearing mouse with nanocomplex at 24 h, 48 h, 72 h, and 96 h post-intravenous injection. | ||
4 Conclusions
We prepared a RES-stealthy nanoprobe with high biocompatibility, stability, and luminescence by producing a noncovalent nanocomplex comprising a hydrophobic dye and albumin. The nanocomplex maintains a stable diameter distribution in both water and saline for several days. The nanocomplex also has a much higher PLQY (0.39) than ICG (0.027 ± 0.005) and is more stable against hydrolysis for more than 14 days. In vitro cellular study demonstrates their ability to label living cells with high biocompatibility even at a high concentration of 368 μM. Although the emission of the hydrophobic dye used in this study is not localized within the wavelength region of the biological window (700–900 nm), the clear in vivo tumor imaging result demonstrates that the nanocomplex is a good nanoprobe for non-invasive tumor diagnosis. To confirm the excellent performance of the nanocomplex for non-invasive tumor imaging, a biodistribution study is further conducted. The results reveal that the exceptional liver- and spleen-stealth property of the nanoprobe enhances the signal-to-noise ratio for tumor diagnosis by reducing the interference of untargeted organs. Long-term tumor imaging for over 4 days is also observed for the nanocomplex, which may enable the monitoring of cancer cell migration and metastasis as well as facilitate the convenient diagnosis post nanoprobe injection. Therefore, this study provides a useful strategy for designing highly biocompatible, luminescent, non-hydrolyzed, and tumor-targetable bioprobes by combining albumin with hydrophobic dyes having a rigid molecular structure. Future researches toward the real clinical applications desire the design and synthesis of safe NIR dyes with high PLQY at aggregated state.Acknowledgements
This work was supported by National Natural Science Foundation of China (no. 91027021, 81171455, 61078073, 31225009), and National Basic Research Program of China (973 Program, Grant Nos. 2010CB934500, 2012CB932400, 2011CB808400, 2011CB707502).References
- P. L. Rodriguez, T. Harada, D. A. Christian, D. A. Pantano, R. K. Tsai and D. E. Discher, Science, 2013, 339, 971 CrossRef CAS PubMed.
- L. Brannon-Peppas and J. O. Blanchette, Adv. Drug Delivery Rev., 2004, 56, 1649 CrossRef CAS PubMed.
- D. Peer, J. M. Karp, S. Hong, O. C. FaroKhzad, R. Margalit and R. Langer, Nat. Nanotechnol., 2007, 2, 751 CrossRef CAS PubMed.
- I. Ozcan, F. Segura-Sanchez, K. Bouchemal, M. Sezak, O. Ozer, T. Guneri and G. Ponchel, Int. J. Nanomed., 2010, 5, 1103 CrossRef PubMed.
- J. Liu, M. Yu, X. Ning, C. Zhou, S. Yang and J. Zheng, Angew. Chem. Int. Ed., 2013, 52, 12804 CrossRef.
- L. L. Xie, W. J. Tong, D. H. Yu, J. Q. Xu, J. Li and C. Y. Gao, J. Mater. Chem., 2012, 22, 6053 RSC.
- J. V. Jokerst, T. Lobovkina, R. N. Zare and S. S. Gambhir, Nanomedicine, 2011, 6, 715 CrossRef CAS PubMed.
- T. Niidome, M. Yamagata, Y. Okamoto, Y. Akiyama, H. Takahashi, T. Kawano, Y. Katayaman and Y. Niidome, J. Controlled Release, 2006, 114, 343 CrossRef CAS PubMed.
- S. T. Yang, K. A. S. Fernando, J. H. Liu, J. Wang, H. F. Sun, Y. F. Liu, M. Chen, Y. P. Huang, X. Wang, H. F. Wang and Y. P. Sun, Small, 2008, 4, 940 CrossRef CAS PubMed.
- P. J. Bonitatibus Jr, A. S. Torres, B. Kandapallil, B. D. Lee, G. D. Goddard, R. E. Colborn and M. E. Marino, ACS Nano, 2012, 6, 6650 CrossRef PubMed.
- M. Cavadas, A. Gonzalez-Fernandez and R. Franco, Nanomedicine, 2011, 7, 730 CAS.
- F. Kratz, J. Controlled Release, 2008, 132, 171 CrossRef CAS PubMed.
- A. O. Elzoghby, W. M. Samy and N. A. Elgindy, J. Controlled Release, 2012, 161, 38 CrossRef CAS PubMed.
- L.-L. Li, P. Wu, K. Hwang and Y. Lu, J. Am. Chem. Soc., 2013, 135, 2411 CrossRef CAS PubMed.
- G. M. Gong, Y. Xu, Y. Y. Zhou, Z. J. Meng, G. Y. Ren, Y. Zhao, X. Zhang, J. H. Wu and Y. Q. Hu, Biomacromolecules, 2012, 13, 23 CrossRef CAS PubMed.
- S. Bae, K. Ma, T. H. Kim, E. S. Lee, K. T. Oh, E. S. Park, K. C. Lee and Y. S. Youn, Biomaterials, 2012, 33, 1536 CrossRef CAS PubMed.
- T. E. McCann, N. Kosaka, M. Mitsunaga, P. L. Choyke, J. C. Gildersleeve and H. Kobayashi, Bioconjugate Chem., 2010, 21, 1925 CrossRef CAS PubMed.
- R. Z. Xu, M. Fisher and R. L. Juliano, Bioconjugate Chem., 2011, 22, 870 CrossRef CAS PubMed.
- A. Bunschoten, T. Buckle, J. Kuil, G. D. Luker, K. E. Luker, O. E. Nieweg and F. W. B. van Leeuwen, Biomaterials, 2012, 33, 867 CrossRef CAS PubMed.
- W. Qin, D. Ding, J. Z. Liu, W. Z. Yuan, Y. Hu, B. Liu and B. Z. Tang, Adv. Funct. Mater., 2012, 22, 771 CrossRef CAS.
- H. W. Sung, D. M. Huang, W. H. Chang, L. L. H. Huang, C. C. Tsai and I. L. Liang, J. Biomater. Sci., Polym. Ed., 1999, 10, 751 CrossRef CAS PubMed.
- B. S. Liu, C. H. Yao, S. H. Hsu, T. S. Yeh, Y. S. Chen and S. T. Kao, J. Biomater. Appl., 2004, 19, 21 CrossRef CAS PubMed.
- K. M. Omer, S. Y. Ku, J. Z. Cheng, S. H. Chou, K. T. Wong and A. J. Bard, J. Am. Chem. Soc., 2011, 133, 5492 CrossRef CAS PubMed.
- C. F. Zheng, M. B. Zheng, P. Gong, D. X. Jia, P. F. Zhang, B. H. Shi, Z. H. Sheng, Y. F. Ma and L. T. Cai, Biomaterials, 2012, 33, 5603 CrossRef CAS PubMed.
- K. Yang, S. Zhang, G. Zhang, X. Sun, S.-T. Lee and Z. Liu, Nano Lett., 2010, 10, 3318 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra47058j |
| This journal is © The Royal Society of Chemistry 2014 |