Rational design of CuO@Cu nanostructure with tuneable morphology and electrochemical properties†
Abstract
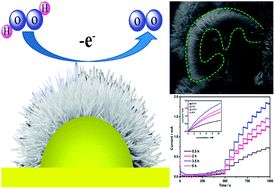
We report the fabrication of novel CuO@Cu nanostructure with tuneable morphology and electrochemical properties. An H2O2 sensor with tuneable sensing features and promising electro-oxidation of MeOH at the CuO@Cu electrode are demonstrated. The constructed H2O2 sensor based on the optimized CuO@Cu electrode has a detection limit of 11 μM.

 Please wait while we load your content...
Please wait while we load your content...