A facile low temperature (350 °C) synthesis of Cu2O nanoparticles and their electrocatalytic and photocatalytic properties†
Abstract
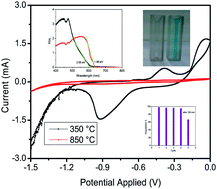
We have synthesized Cu2O nanoparticles (∼25 nm) starting from CuO (∼107 nm) and copper oxalate nanorods in an inert atmosphere (Ar) at a very low temperature of 350 °C. The process in the absence of the oxalate nanorods yields Cu2O at a much higher temperature of 850 °C. The Cu2O synthesized at lower temperature (350 °C) has smaller particles than Cu2O synthesized at 850 °C. We explored these nanomaterials as electrocatalysts for hydrogen and oxygen evolution which are highly desirable for renewable and clean energy applications. Electrochemical studies such as the hydrogen evolution reaction and oxygen evolution reaction (HER & OER) were carried out on glassy carbon as well as on platinum as the working electrode in KOH solution. Cu2O synthesized at lower temperature (350 °C) has 14 times higher current density during HER and 2 times higher current density during OER. These electrocatalysts were stable for 50 cycles. However, the Cu2O synthesized at higher temperature (850 °C) showed very efficient (∼98%) degradation of methylene blue (MB) in 120 min and the catalyst is stable up to the 4th cycle whereas Cu2O (350 °C) shows only 40% degradation.

 Please wait while we load your content...
Please wait while we load your content...