Electrochemically reduced graphene oxide with porous structure as a binder-free electrode for high-rate supercapacitors†
Xuejun Liuab,
Xiang Qi*ab,
Zhen Zhangab,
Long Renab,
Guolin Haoab,
Yundan Liuab,
Yao Wangab,
Kai Huangab,
Xiaolin Weiab,
Jun Liab,
Zongyu Huangab and
Jianxin Zhongab
aFaculty of Materials and Optoelectronic Physics, Xiangtan University, Hunan 411105, PR China. E-mail: xqi@xtu.edu.cn; Tel: +86-0731-58292195
bHunan Provincial Key Laboratory of Micro-Nano Energy Materials and Devices, Laboratory for Quantum Engineering and Micro-Nano Energy Technology, Xiangtan University, Hunan 411105, PR China
First published on 4th March 2014
Abstract
A binder-free electrode is prepared by directly depositing electrochemically reduced graphene oxide (ERGO) on the metal current collector. Fourier transform infrared spectroscopy and Raman spectrum have been used to demonstrate the effective reduction of graphene oxide on the electrode, and the porous structure of the ERGO film was further characterized by scanning electron microscopy. The electrochemical properties of ERGO were investigated by cyclic voltammetry, galvanostatic charge–discharge and electrochemical impedance spectroscopy (EIS). Electrochemical measurements showed that the binder-free ERGO electrode had high specific capacity, good cycle stability, as well as excellent high-rate capability. The specific capacitance of the constructed electrode was 131.6 F g−1 at a scan rate of 10 mV s−1 and maintained 66.9% of the initial value when the scan rate was increased up to 1000 mV s−1. Owing to its favorable electrochemical performance, this binder-free ERGO electrode with porous structure has great potential in future commercial electrochemical supercapacitors.
1. Introduction
Graphene, as an one-atom-thick sheet of sp2-bonded carbon atoms arranged in a honeycomb structure, has emerged as a new class of promising materials, which are attractive for potential applications in photonics, optoelectronics, supercapacitors and batteries.1–5 Significantly, considerable efforts have been devoted to exploitation of graphene as the supercapacitor electrode material owing to its superior properties such as high electrical conductivity, large specific surface area, great mechanical strength and excellent electrochemical stability.6–8 Many methods have been developed to produce graphene such as mechanical exfoliation,9 chemical vapor deposition (CVD)10 and epitaxial growth.11 Thereinto, oxidative exfoliation of natural graphite followed by reduction12 is regarded as the most promising approach for mass production of graphene. Up to now, graphene oxide (GO) can be reduced by different routes including thermal,13 chemical,14 microwave-assisted15 and electrochemical16 process. Considering the poisonous reducing agents introduced or high temperature needed in the process, which may cause serious environmental issues and large energy consumption, a green electrochemical method requiring no hazardous reductants is highly desirable.17 Most recently, electrochemically reduced graphene oxide (ERGO) has been successfully synthesized. It has been reported that ERGO exhibits superior capacitive performance.18–20 However, the ERGO has been not yet integrated with the metal current collectors.Generally, the EDLC electrode is fabricated by coating the mixture of electrode materials and binders onto the metal current collector.21–24 The use of polymer binder in the electrode is to bind active materials with the conducting additives to the collectors. Increasing the amount of polymer binder increases the adhesion strength of the electrode but at the expense of the electrode's conductivity.25 In contrast, the binder-free electrode reduces harmful effects of the polymeric binder, weight of the active material and simplifies the electrode preparation process. For example, Chen26 reported fabrication of CNT directly grown on graphite foil by CVD technique using nickel as catalyst. A high specific capacitance of 115.7 F g−1 and rectangular-shaped cyclic voltammetric curves were obtained when the scan rate increased to 100 mV s−1. Emmenegger27 deposited CNTs on silicon or aluminum by CVD technique with Fe(NO3)3 as catalyst and achieved capacity of 120 F cm−3. Park28 produced CNTs on the stainless steel by a sequential combination of plasma enhanced CVD and thermal CVD, and the binder-free electrode showed specific capacitance in the range of 33–82 F g−1. Yoon29 synthesized CNT on the nickel substrates and the cyclic voltammetry (CV) of CNT electrode maintained rectangular even at 1000 mV s−1. In view of graphene, while CVD technique enables direct growth of graphene with high quality, the low throughput, high temperature, high-quality substrate materials and accurate control over cooling rates involved in the process render the CVD graphene impractical for large-scale manufacturing,30 which limit its potential as the electrode material. Recently, electrochemical reduction of graphene oxide has been studied.19,20,31 However, the layered structure of the ERGO may restrict the diffusion of ions and provide less active surface area when the ERGO is used as the electrode for the supercapacitor.
In this study, ERGO is immediately deposited on the stainless steel from GO dispersion and used as the binder-free electrode. The proposed method requires no binders and simplifies the fabrication procedure of the electrode. The constructed binder-free electrode exhibits excellent rate performance compared with binder-containing electrode, which is due to the porosity of ERGO film, as well as the minimal contact resistance between the electrode materials and current collector.
2. Experimental section
2.1 Materials and methods
GO was synthesized from graphite powder by the modified Hummers' method.32 A total of 125 mg GO was mixed with 50 mL de-ionized water and ultrasonicated for 1 h to yield a stable GO suspension (2.5 mg mL−1). Typically, the ERGO electrode was fabricated by electrochemical reduction of GO suspension maintaining at 60 °C under a constant potential of −1.0 V (vs. Ag/AgCl) for 2 hours. A stainless steel was used as working electrode with Pt foil and Ag/AgCl electrode as counter and reference electrodes, respectively. Prior to electrochemical synthesis, the stainless steel was polished with emery paper and rinsed by ultrasonication in acetone, ethanol and de-ionized water. In order to improve the conductivity, the as-prepared ERGO was further reduced for another 60 s in 1 M Na2SO4 aqueous solution. For comparison, the ERGO deposited on the electrode was peeled off and mixed with a common binder of poly (vinylidene fluoride) (PVDF) in ethanol with the mass ratio of 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15. The mixture was ground in an agate mortar and then pressed onto the stainless steel. Finally, the as-prepared electrode denoted as ERGO–PVDF was dried in air as the controlled binder-containing electrode.
15. The mixture was ground in an agate mortar and then pressed onto the stainless steel. Finally, the as-prepared electrode denoted as ERGO–PVDF was dried in air as the controlled binder-containing electrode.
2.2 Characterization
The surface morphologies and microstructures of as-deposited electrode were characterized by Scan Electron Microscopy (SEM, JEOL JSM-6360), Fourier transform infrared spectroscopy (FTIR, PE Spectrum One) and Raman spectroscopy (Raman, Renishaw 100). The electrochemical properties of the supercapacitor were measured in 1 M Na2SO4 aqueous electrolyte by a CHI 660D electrochemical workstation.3. Results and discussion
Fig. 1 is the typical SEM images of the conformation of ERGO. It is clear seen that the wrinkling and crumpling framework with porous structure is deposited on the stainless steel collector. Crumpled construction can effectively prevent GO from restacking and the porous configuration could deliver much higher specific capacitance and better rate capability.33,34 Further characterizations are carried out by FTIR and Raman spectra. FTIR spectra (Fig. 2(a)) is recorded to confirm the chemical structure of the as-prepared sample, and the following functional groups are identified35 in the GO sample: the strong peak of 1730 cm−1 attributes to the C–O stretching vibrations of the COOH groups, the peak of 1620 cm−1 assigns to the contributions from the skeletal vibrations of the graphitic domains, the peaks at 1384 and 1224 cm−1 belong to the C–OH stretching vibrations, and the other peaks are due to –OH, C–O and C–O–C stretching at 3392, 1052 and 856 cm−1, respectively. After electrochemical deposition, these characteristic peaks of GO decrease obviously, which prove the effectiveness of the reduction process. Raman measurements are also used to characterize pristine GO and ERGO samples. As shown in Fig. 2(b), the Raman spectrum of GO displays two prominent peaks at 1355 and 1590 cm−1, which correspond to the well-documented D and G bands, respectively. Although the Raman spectrum of ERGO samples also have both D and G bands at 1355 and 1590 cm−1, the intensity ratio of D/G increased significantly in comparison with that of the GO. This change in the intensity ratio of the D to G bands is attributed to the increased defect concentration present in ERGO relative to that in GO, indicating a decrease in the average size of the sp2 domains upon reduction of the exfoliated GO, and can be explained by creation of new graphitic domains that are smaller in size than the ones presented in GO.36 | ||
| Fig. 1 (a) and (b) Scanning electron microscope images of electrochemically reduced graphene oxide (ERGO) (inset is the optical image of ERGO on stainless steel electrode). | ||
 | ||
| Fig. 2 (a) FTIR spectra of ERGO (red line) and pristine graphene oxide (black line); (b) Raman spectra of ERGO (red line) and pristine graphene oxide (black line). | ||
In order to investigate the influence of potential on the electrosynthesis of ERGO, GO suspension is electrochemically reduced under various potentials between −1 and 1 V. The results demonstrate that a black layer of ERGO is successfully deposited onto the stainless steel in the potential range of −0.7 to −1 V. CV is also adopted to investigate the electrochemical properties of GO dispersion. The CV curve of GO on the stainless steel is shown in Fig. S1(a).† A large cathodic current wave at −1.0 V with an onset at −0.6 V was observed. This wave is attributed to the reduction of the oxygen-containing groups (hydroxyl and epoxy groups) on GO sheets.37 Therefore, GO can be reduced at potential lower than −0.6 V according to the CV curve. In order to maximize the reaction rate and minimize the decomposition of water, the electrochemical reduction is performed at −1.0 V. Furthermore, it is also found that the increased reduction temperature could further accelerate the reaction rate and defects may be effectively eliminated in this way.38
Deposition time is another factor affecting the fabrication of ERGO electrode. For comparison, the controlled electrodes are electrochemically produced for 0.5, 1, 2 and 4 hours. SEM images of the electrodes with different deposition times are illustrated in Fig. 3. When the deposition time reached 30 minutes, individual small graphene clusters are formed and randomly distributed on the electrode. As the deposition time increases to 1 hour, isolated clusters coalesce and interpenetrating graphene microstructure is formed. After 2 hours, the ERGO sheets self-assemble with each other and crumpled, interconnecting networks are constructed. The porous structure remains unchanged when the time increases to 4 hours. The morphological evolution is proposed to be derived from competition between the effect of deposition driving the system out-of-equilibrium, and equilibrating surface diffusion processes, which is similar with the kinetic process of 2D cluster vapor or solution phase growth.39,40
The electrochemical impedance spectroscopy is also used to examine the performance of the ERGO based electrode with different deposition times. As is shown in Fig. S1(b),† the ERGO fabricated for 30 minutes exhibits nearly a vertical line, reflecting its high conductivity and rate capability. As the deposition time increases to 1 hour, a semicircular part in the high-frequency region, followed by a straight line in the low-frequency region can be observed. The high frequency loop is related to the electronic resistance. It's clear from the plot that the resistances of the electrodes increase with the extension of deposition time. Although ERGO synthesized for 0.5 and 1 hour displayed relatively low resistances, its mass is so small that ERGO exhibits low specific capacitance evaluated in area units. In contrast, when ERGO is synthesized for 4 hours, its mass per unit area is large. However, its resistance was relatively high, which was detrimental to the high-rate performance. Therefore, the electrochemical reduction would be performed for 2 hours to obtain high specific capacitance as well as high-rate capability.
Directly using the ERGO synthesized for 2 hours on the metal current collector as binder-free electrode, the electrochemical performances are firstly evaluated by means of CV and charge–discharge tests in a typical three-electrode system. Meanwhile, a similar set-up with ERGO based electrode replaced by binder-containing electrode is adopted for comparison. Fig. 4(a) is the CV curves for ERGO based electrode under different scan rates. From the curves, it can be found that the electrode exhibits fairly rectangular shape without obvious redox peaks at all scan rates from 10 mV s−1 to 100 mV s−1, displaying good capacitive behavior at the electrode surface following the electric double layer charging mechanism.41 In the case of ERGO–PVDF based electrode (Fig. S2(a)†), CV profiles still maintain a relatively rectangular shape with the increasing potential scan rates. While both types of electrodes display excellent capacitive performance under a low scan rate (Fig. S2(b)†), ERGO based electrode retains a fair well rectangular shape even at a high scan rate 10 times larger than that in Fig. S2(b)† in contrast to ERGO–PVDF modified electrode, which shows a big distortion at the same scan rate, as shown in Fig. 4(b). These results imply that ERGO based electrode holds superior high-rate capability compared with ERGO–PVDF modified electrode.
In order to further assess the rate performance, the CV measurements are carried out at different scan rates from 100 to 1000 mV s−1 for the as-prepared ERGO and comparative tests based on ERGO–PVDF are also implemented. As is illustrated in Fig. 4(c), the CV curves of ERGO are in typical rectangular shape even when the scan rate increases up to 1000 mV s−1, suggesting that the ERGO has an outstanding high-rate performance. On the contrary, the shape of CV curves of ERGO–PVDF (Fig. 4(d)) become narrower and more oblique as the scan rate increases. The specific capacitances of ERGO and ERGO–PVDF calculated from CV curves are estimated according to eqn (1) (ref. 42) and recorded as a function of scan rates.
 | (1) |
| Type of materials | Binder | Electrolyte | High scan rates (mV s−1) | Specific capacitance (F g−1) | Capacitance retention (%) | Ref. |
|---|---|---|---|---|---|---|
| CMG | PTFE | 5.5 M KOH | 400 | 97 | 96 | 43 |
| GNS | Free | 6 M KOH | 100 | 97 | 60 | 44 |
| FGS | PTFE | 2 M KOH | 100 | 108 | 48 | 45 |
| RGO | Free | 0.5 M Na2SO4 | 500 | 40–60 | 40–75 | 46 |
| ERGO–PVDF | PVDF | 1 M Na2SO4 | 200 | 14.6 | 17.7 | This work |
| ERGO | Free | 1 M Na2SO4 | 1000 | 88 | 66.9 | This work |
Fig. 5(a) and S3† demonstrate the representative galvanostatic charge–discharge curves of ERGO and ERGO–PVDF at a charging–discharging current density of 0.5, 1, 2 and 5 mA cm−2 under an applied potential between −1.0 and 0 V. As shown in Fig. 5(a), the charge–discharge plots of ERGO electrode exhibit the near linear and symmetric triangles, indicating its superior capacitive performance.42 Moreover, the iR drops on all curves are too small to identify even at a current density of 5 mA cm−2, implying small internal resistance of ERGO based electrode. In contrast, an obvious iR drops (marked by the circle in Fig. S3†) at the very beginning of the discharge process are obtained and became sharply as the current density increased, which is caused by relatively large overall internal resistance of ERGO–PVDF based electrode. The specific capacitances measured from galvanostatic charge–discharge curves are estimated according to eqn (2).42
 | (2) |
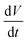 is the slope of the discharge curve. The specific capacitance of ERGO calculated from Fig. 5(a) is 126.4 F g−1 at a current density of 0.5 mA cm−2, and the value is comparable to that of ERGO–PVDF (108.6 F g−1, Fig. S3†). However, as is shown in Fig. 5(b), the ERGO–PVDF based electrode has lost its 67.2% capacitance as the current density increased from 0.5 to 5 mA cm−2, while ERGO based electrode still maintains 78.6% specific capacitance.
is the slope of the discharge curve. The specific capacitance of ERGO calculated from Fig. 5(a) is 126.4 F g−1 at a current density of 0.5 mA cm−2, and the value is comparable to that of ERGO–PVDF (108.6 F g−1, Fig. S3†). However, as is shown in Fig. 5(b), the ERGO–PVDF based electrode has lost its 67.2% capacitance as the current density increased from 0.5 to 5 mA cm−2, while ERGO based electrode still maintains 78.6% specific capacitance.
In order to further evaluate the cycling stability of ERGO, galvanostatic charge–discharge studies are performed at a constant current density of 0.5 mA cm−2 between −1 and 0 V in 1 M Na2SO4 electrolyte. As shown in Fig. 5(c), the specific capacitance of ERGO based electrode remains 90.3% of the initial capacitance, reflecting that the binder-free electrode has good electrochemical stability and a high degree of reversibility upon repetitive charge–discharge process. To confirm the advantage of the constructed electrode, the electrochemical impedance spectroscopy is also examined with a frequency range from 100 mHz to 100 kHz using an AC perturbation amplitude of 10 mV. As shown in Fig. 5(d), the Nyquist plots of ERGO based supercapacitor display a straight line in the low-frequency region and an arc in the high frequency region. The vertical line in the low frequency region, corresponding to a diffusion-limiting process, indicates a pure capacitive behavior. The more vertical the curve, the more closely the supercapacitor behaves as an ideal capacitor.47 In the high frequency region, the intercept of the semicircle with the real axis represents the equivalent series resistance (ESR) containing the resistance of the electrolyte solution, the intrinsic resistance of the active materials, and the contact resistance of the interface active material/current collector. The ESR value of ERGO based supercapacitor is 0.86 ohm, which is a small value and determines the rate that can be charged/discharged.48 The semicircular part in the high frequency is related to the electronic resistance between graphene sheets. Compared with ERGO, the size of the semicircle for ERGO–PVDF is larger, which might be probably resulted from the addition of PVDF binder in the electrode materials.49
4. Conclusion
A simple electrochemical synthesis method has been developed to prepare ERGO based film with porous structure. The ERGO is directly deposited on the stainless steel and used as a binder-free electrode for the supercapacitor. The formation mechanism for the growth of ERGO on the current collector has been investigated. Influence of factors such as potential and time on the electrochemical deposition of graphene had also been explored. When tested in 1 M Na2SO4 solution, the constructed electrode shows much higher electrochemical performances in terms of specific capacitance, rate capability and EIS compared with the controlled electrode made by the mixture of ERGO and PVDF. The cycling performance of ERGO is also evaluated, maintaining 90.3% of the initial capacitance. The results indicate that the binder-free electrode possesses superior cycling stability. The excellent performances of the binder-free electrode can be attributed to the porous character of ERGO film, as well as the low contact resistance between the electrode materials or between the electrode materials and the current collector. Our results propose that this binder-free ERGO electrode with favorable electrochemical properties is a competitive candidate for electrochemical energy storage.Acknowledgements
This work was supported by the Grants from National Natural Science Foundation of China (nos 51002129, 51172191, 11204262 and 11074211), National Basic Research Program of China (2012CB921303), Research Fund for the Doctoral Program of Higher Education of China (no. 20124301120006), Hunan Science and Technology Bureau planned project (no. 2012SK3166), Open Fund based on innovation platform of Hunan colleges and universities (no. 12K045 and 13K045) and the China Postdoctoral Science Foundation funded project (no. 20100480068).References
- M.-Y. Yen, C.-K. Hsieh, C.-C. Teng, M.-C. Hsiao, P.-I. Liu, C.-C. M. Ma, M.-C. Tsai, C.-H. Tsai, Y.-R. Lin and T.-Y. Chou, RSC Adv., 2012, 2, 2725–2728 RSC.
- X. Li and C. Wang, J. Mater. Chem. A, 2013, 1, 165–182 CAS.
- X. Li, Y. Hu, J. Liu, A. Lushington, R. Li and X. Sun, Nanoscale, 2013, 5, 12607–12615 RSC.
- S. Bai and X. Shen, RSC Adv., 2012, 2, 64–98 RSC.
- X. Li, D. Geng, Y. Zhang, X. Meng, R. Li and X. Sun, Electrochem. Commun., 2011, 13, 822–825 CrossRef CAS PubMed.
- Y. Huang, J. Liang and Y. Chen, Small, 2012, 8, 1805–1834 CrossRef CAS PubMed.
- X. Yang, C. Cheng, Y. Wang, L. Qiu and D. Li, Science, 2013, 341, 534–537 CrossRef CAS PubMed.
- L. Sun, L. Wang, C. Tian, T. Tan, Y. Xie, K. Shi, M. Li and H. Fu, RSC Adv., 2012, 2, 4498–4506 RSC.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- G. Kalita, M. S. Kayastha, H. Uchida, K. Wakita and M. Umeno, RSC Adv., 2012, 2, 3225–3230 RSC.
- P. W. Sutter, J.-I. Flege and E. A. Sutter, Nat. Mater., 2008, 7, 406–411 CrossRef CAS PubMed.
- G. Eda and M. Chhowalla, Adv. Mater., 2010, 22, 2392–2415 CrossRef CAS PubMed.
- M. J. McAllister, J.-L. Li, D. H. Adamson, H. C. Schniepp, A. A. Abdala, J. Liu, M. Herrera-Alonso, D. L. Milius, R. Car, R. K. Prud'homme and I. A. Aksay, Chem. Mater., 2007, 19, 4396–4404 CrossRef CAS.
- P. Song, X. Zhang, M. Sun, X. Cui and Y. Lin, RSC Adv., 2012, 2, 1168–1173 RSC.
- X. Liu, L. Pan, T. Lv, G. Zhu, T. Lu, Z. Sun and C. Sun, RSC Adv., 2011, 1, 1245–1249 RSC.
- M. F. Hossain and J. Y. Park, RSC Adv., 2013, 3, 16109–16115 RSC.
- S. Pei and H.-M. Cheng, Carbon, 2012, 50, 3210–3228 CrossRef CAS PubMed.
- G. Zhao, T. Wen, C. Chen and X. Wang, RSC Adv., 2012, 2, 9286–9303 RSC.
- H. Yu, J. He, L. Sun, S. Tanaka and B. Fugetsu, Carbon, 2013, 51, 94–101 CrossRef CAS PubMed.
- P. Si, S. Ding, X.-W. Lou and D.-H. Kim, RSC Adv., 2011, 1, 1271–1278 RSC.
- K.-S. Kim and S.-J. Park, Electrochim. Acta, 2012, 78, 147–153 CrossRef CAS PubMed.
- S. Zhang, Y. Li and N. Pan, J. Power Sources, 2012, 206, 476–482 CrossRef CAS PubMed.
- Y. Zhang, F. Xu, Y. Sun, Y. Shi, Z. Wen and Z. Li, J. Mater. Chem., 2011, 21, 16949–16954 RSC.
- P. Staiti, A. Arenillas, F. Lufrano and J. Á. Menéndez, J. Power Sources, 2012, 214, 137–141 CrossRef CAS PubMed.
- M.-S. Wu and K.-H. Lin, J. Phys. Chem. C, 2010, 114, 6190–6196 CAS.
- J. H. Chen, W. Z. Li, D. Z. Wang, S. X. Yang, J. G. Wen and Z. F. Ren, Carbon, 2002, 40, 1193–1197 CrossRef CAS.
- C. Emmenegger, P. Mauron, A. Züttel, C. Nützenadel, A. Schneuwly, R. Gallay and L. Schlapbach, Appl. Surf. Sci., 2000, 162–163, 452–456 CrossRef CAS.
- D. Park, Y. Hoon Kim and J. Kee Lee, Carbon, 2003, 41, 1025–1029 CrossRef CAS.
- B.-J. Yoon, S.-H. Jeong, K.-H. Lee, H. Seok Kim, C. Gyung Park and J. Hun Han, Chem. Phys. Lett., 2004, 388, 170–174 CrossRef CAS PubMed.
- S. Guo and S. Dong, Chem. Soc. Rev., 2011, 40, 2644–2672 RSC.
- J. Yang and S. Gunasekaran, Carbon, 2013, 51, 36–44 CrossRef CAS PubMed.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- J. Luo, H. D. Jang and J. Huang, ACS Nano, 2013, 7, 1464–1471 CrossRef CAS PubMed.
- L. Zhang and G. Shi, J. Phys. Chem. C, 2011, 115, 17206–17212 CAS.
- J. Xu, K. Wang, S.-Z. Zu, B.-H. Han and Z. Wei, ACS Nano, 2010, 4, 5019–5026 CrossRef CAS PubMed.
- S. Stankovich, D. A. Dikin, R. D. Piner, K. A. Kohlhaas, A. Kleinhammes, Y. Jia, Y. Wu, S. T. Nguyen and R. S. Ruoff, Carbon, 2007, 45, 1558–1565 CrossRef CAS PubMed.
- K. Chen, L. Chen, Y. Chen, H. Bai and L. Li, J. Mater. Chem., 2012, 22, 20968–20976 RSC.
- H.-L. Guo, X.-F. Wang, Q.-Y. Qian, F.-B. Wang and X.-H. Xia, ACS Nano, 2009, 3, 2653–2659 CrossRef CAS PubMed.
- I. Doudevski, W. A. Hayes and D. K. Schwartz, Phys. Rev. Lett., 1998, 81, 4927–4930 CrossRef CAS.
- J. W. Evans, P. A. Thiel and M. C. Bartelt, Surf. Sci. Rep., 2006, 61, 1–128 CrossRef CAS PubMed.
- X. Xin, X. Zhou, F. Wang, X. Yao, X. Xu, Y. Zhu and Z. Liu, J. Mater. Chem., 2012, 22, 7724–7730 RSC.
- V. H. Luan, H. N. Tien, L. T. Hoa, N. T. M. Hien, E.-S. Oh, J. Chung, E. J. Kim, W. M. Choi, B.-S. Kong and S. H. Hur, J. Mater. Chem. A, 2013, 1, 208–211 CAS.
- M. D. Stoller, S. Park, Y. Zhu, J. An and R. S. Ruoff, Nano Lett., 2008, 8, 3498–3502 CrossRef CAS PubMed.
- Y. Chen, X. Zhang, P. Yu and Y. Ma, J. Power Sources, 2010, 195, 3031–3035 CrossRef CAS PubMed.
- Q. Du, M. Zheng, L. Zhang, Y. Wang, J. Chen, L. Xue, W. Dai, G. Ji and J. Cao, Electrochim. Acta, 2010, 55, 3897–3903 CrossRef CAS PubMed.
- Y. J. Oh, J. J. Yoo, Y. I. Kim, J. K. Yoon, H. N. Yoon, J.-H. Kim and S. B. Park, Electrochim. Acta, 2014, 116, 118–128 CrossRef CAS PubMed.
- Y. Wang, Z. Shi, Y. Huang, Y. Ma, C. Wang, M. Chen and Y. Chen, J. Phys. Chem. C, 2009, 113, 13103–13107 CAS.
- C. Liu, Z. Yu, D. Neff, A. Zhamu and B. Z. Jang, Nano Lett., 2010, 10, 4863–4868 CrossRef CAS PubMed.
- W.-C. Chen, T.-C. Wen and H. Teng, Electrochim. Acta, 2003, 48, 641–649 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra46992a |
| This journal is © The Royal Society of Chemistry 2014 |



