Ship in a bottle synthesis of ionic liquids in NaY supercages for CO2 capture
Abstract
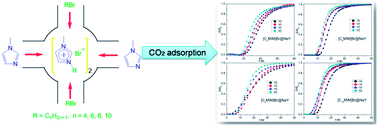
CO2 is the single most important anthropogenic greenhouse gas, contributing ∼64% to the global radiative forcing. And the rising concentration of CO2 in the atmosphere will result in global climate change. In this study, 1-alkyl-3-methylimidazolium bromide ionic liquids (ILs) ([CnMIM]Br, n = 4, 6, 8, 10) were ship in a bottle synthesized in NaY zeolite to get [CnMIM]Br@NaY samples and applied for CO2 capture. These samples were then characterized by elemental analysis, thermal gravimetric analysis (TGA), X-ray diffraction (XRD) and FT-Raman spectra. The results indicated that [CnMIM]Br ILs were successfully encapsulated inside NaY and the encapsulated [CnMIM]Br ILs were much more stable than their bulk analogues. And Raman spectra showed that the relative intensities of some peaks in the [CnMIM]Br@NaY samples had good relationships with the side chain length of ILs. Then the breakthrough curves were recorded to evaluate the CO2 adsorption capacity of these samples, and indicated that the highest adsorption capacity could reach up to 20.08 mL CO2 per g [C4MIM]Br@NaY. And the cyclic CO2 adsorption results also illustrated that the [CnMIM]Br@NaY samples were stable and effective with prolonged use. So these samples could be potential materials for CO2 capture.

 Please wait while we load your content...
Please wait while we load your content...