Plasma and brain pharmacokinetics of previously unexplored lithium salts†
Abstract
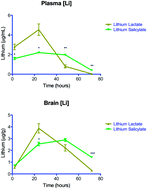
Despite its narrow therapeutic window, lithium is still regarded as the gold standard comparator and benchmark treatment for mania. Recent attempts to find new drugs with similar therapeutic activities have yielded new chemical entities. However, these potential new drugs have yet to match the many bioactivities attributable to lithium's efficacy for the treatment of neuropsychiatric diseases. Consequently, an intense effort for re-engineering lithium therapeutics using crystal engineering is currently underway. We sought to improve the likelihood of success of these endeavors by evaluating the pharmacokinetics of previously unexplored lithium salts with organic anions (lithium salicylate and lithium lactate). We report that these lithium salts exhibit profoundly different pharmacokinetics compared to the more common FDA approved salt, lithium carbonate, in rats. Remarkably, lithium salicylate produced elevated plasma and brain levels of lithium beyond 48 hours post-dose without the sharp peak that contributes to the toxicity problems of current lithium therapeutics. These findings could be important for the development of the next generation of lithium therapeutics.

 Please wait while we load your content...
Please wait while we load your content...