MoS2–reduced graphene oxide composites synthesized via a microwave-assisted method for visible-light photocatalytic degradation of methylene blue†
Jinliang Lia,
Xinjuan Liub,
Likun Pan*a,
Wei Qina,
Taiqiang Chena and
Zhuo Suna
aEngineering Research Center for Nanophotonics & Advanced Instrument, Ministry of Education, Shanghai Key Laboratory of Magnetic Resonance, Department of Physics, East China Normal University, Shanghai 200062, China. E-mail: lkpan@phy.ecnu.edu.cn; Fax: +86 21 62234321; Tel: +86 21 62234132
bShanghai Nanotechnology Promotion Center, Shanghai 200237, China
First published on 28th January 2014
Abstract
MoS2–reduced graphene oxide (RGO) composites were successfully synthesized via microwave-assisted reduction of graphite oxide in a MoS2 precursor aqueous solution using a microwave system. The morphology, structure and photocatalytic performance in the degradation of methylene blue (MB) were characterized by scanning electron microscopy, X-ray diffraction, electrochemical impedance spectra and UV-vis absorption spectroscopy, respectively. The results show that the MoS2–RGO composites exhibit enhanced photocatalytic performance in the degradation of MB with a maximum degradation rate of 99% under visible light irradiation for 60 min. This excellent photocatalytic activity is due to the contribution from the reduced electron–hole pair recombination, the enhanced light absorption and the increased dye adsorptivity with the introduction of RGO in the composite.
1. Introduction
Considerable attention has been paid to semiconductor photocatalysis for water treatment and splitting due to its efficient destruction and transformation ability.1–9 However, the traditional photocatalysts, such as TiO2 and ZnO, with a wide band gap can only exhibit high photocatalytic activity under ultraviolet light irradiation, which significantly limits their practical applications.10–12 To better utilize the solar light which is composed of about 45% of visible light, it is desirable to exploit novel visible light-sensitive semiconductor photocatalysts.Molybdenum disulfide (MoS2) as a novel semiconductor features a layered structure in which the atoms are covalently bonded to form two-dimensional layers that are stacked together through weak Van der Waals interaction.13–16 MoS2 with a narrow band gap of ∼1.8 eV has a strong absorption in the visible spectrum region, therefore it has been exploited for photocatalytic applications.17–22 James et al.23 obtained the MoS2 nanoparticles through thermally decomposing method for photocatalytic degradation of methylene blue (MB) and a degradation efficiency of ∼30% was achieved. Hu et al.24 synthesized nano-ball, nano-slice and bulk MoS2 by precipitation method and found that nano-slice MoS2 showed higher photocatalytic efficiency (∼90%) in the degradation of methyl orange than others. It should be noticed that due to the quick recombination of photo-generated charge carriers in MoS2,12,25 its photocatalytic efficiency is still to be further improved.
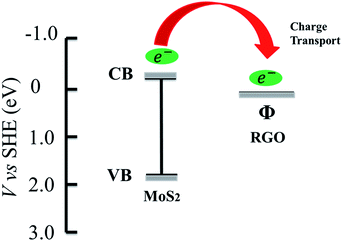
Graphene as an excellent electron-acceptor/transport material has been applied to photocatalysis because it can decrease the photo-generated electron–hole recombination and improve the light adsorption.26–29 Currently, graphene has been introduced into many photocatalysts, such as TiO2 and ZnO, to improve their photocatalytic performance.28,30–33 Similarly, graphene can also be incorporated into MoS2 to improve its photocatalytic performance considering the stepwise structure of energy levels constructed in the MoS2–graphene composite, as shown in Fig. 1. According to the data reported on the conduction band (CB) and valence band (VB) of MoS2 (−0.13 and 1.72 eV vs. NHE)18 and the work function of graphene (−0.08 eV vs. NHE.),34–36 the energy levels are beneficial for photo-induced electrons to transfer from the MoS2 CB to the graphene, which could efficiently separate the photo-induced electrons and hinder the charge recombination in the electron-transfer processes, thus enhance the photocatalytic performance.30 Min et al.22 synthesized the limited-layered MoS2 confined on reduced graphene oxide (RGO) sheets for hydrogen evolution by hydrothermal process and found that MoS2 co-catalyst on RGO sheets exhibits much higher activity than pristine MoS2 particles under visible light irradiation. Despite the above progress, there is few report on the synthesis, especially using a rapid microwave-assisted method,28,37 and investigation of MoS2–RGO composites for the photocatalytic degradation of organics.
In this work, one-step synthesis of MoS2–RGO composites was carried out through microwave-assisted reduction of graphite oxide (GO) dispersion in MoS2 precursor solution using a microwave system, and their photocatalytic performances in the degradation of MB under visible light irradiation were investigated. The MoS2–RGO composites exhibit enhanced photocatalytic performance in the degradation of MB under visible light irradiation compared with pure MoS2.
2. Experimental
2.1. Synthesis
All the reagents were of analytical grade and used without further purification. Commercial graphite powder was used as the starting reagent for the synthesis of GO via modified Hummers method, as described in our previous works.38–40 Different amount of GO was added into 10 mL 0.02 M phosphomolybdic acid hydrate solution and then the pH value was adjusted to 7 by adding 1 M NaOH solution. And then 10 mL 0.72 M thioacetamide solution was added into the solution and stirred for 10 min to produce a uniform dispersion. Subsequently, the mixture solution was placed in a 35 ml microwave tube and then put into an automated focused microwave system (Explorer-48, CEM Co.) and treated at 150 °C with a microwave irradiation power of 150 W for 10 min. The as-synthesized MoS2–RGO samples with 0.25, 0.5, 0.75 and 1 wt% GO, named as MG-1, MG-2, MG-3 and MG-4 were isolated by centrifugation (8000 rpm) for 20 min, washed three times with distilled water and finally dried in a vacuum oven at 80 °C for 24 h. As described in our previous work, most of GO has been reduced to RGO under microwave irradiation and the obtained RGO has a C/O ratio of ∼9.12, which is close to the value (∼10) of that achieved by hydrazine reduction method.39–41 Pure MoS2 and RGO were synthesized by a direct microwave assisted reaction of the MoS2 precursor solution and the GO suspension for comparison. Furthermore, the as-synthesized composites with 5 wt% cellulose binder were homogeneously mixed in terpineol to form a slurry. Then, the resultant slurries were coated on fluorine-doped tin oxide (FTO) glass substrate for the electrochemical impedance spectra (EIS) testing and quartz glass slide for the UV-vis absorption spectra testing using a screen-printing approach, respectively.2.2. Characterization
The surface morphology and structure of the samples were characterized by field-emission scanning electron microscopy (FESEM, Hitachi S-4800), X-ray diffraction (XRD, Holland Panalytical PRO PW3040/60) with Cu-Kα radiation (V = 30 kV, I = 25 mA) and energy dispersive X-ray spectroscopy (EDS, JEM-2100), respectively. The UV-vis absorption spectra were recorded using a Hitachi U-3900 UV-vis spectrophotometer. EIS measurement was carried out on an electrochemical workstation (AUTOLAB PGSTAT302N) under dark conditions using a three electrode configuration with the as-prepared films as working electrodes, a Pt foil as counter electrode and a standard calomel electrode as reference electrode. The electrolyte was 60 mg L−1 methylene blue (MB) aqueous solution. EIS were recorded in the frequency range of 0.1 Hz to 1 MHz, and the applied bias voltage and ac amplitude were set at open-circuit voltage and 10 mV.2.3. Photocatalytic experiments
The photocatalytic performances of the as-prepared samples were evaluated through the photocatalytic degradation of MB under visible light irradiation. The samples (80 mg) were dispersed in 80 ml MB aqueous solutions (60 mg L−1). The suspensions were first treated ultrasonically for 5 min and then magnetically stirred in the dark for 30 min to reach the adsorption–desorption equilibrium. Under ambient conditions and stirring, the suspensions were exposed to visible light irradiation produced by a 5 W white LED light lamp with the main wave crest at 450 and 558 nm and a color temperature of 5750 K. At certain time intervals, 2 ml of the suspensions were extracted and centrifuged to remove the photocatalyst. The filtrates were analyzed by recording the UV-vis spectra of MB using a Hitachi U-3900 UV-vis spectrophotometer.3. Results and discussion
Fig. 2(a) and (b) show the FESEM images of RGO and MoS2. The RGO nanosheets are curled and corrugated and MoS2 displays scaled sheets which are stacked together. Fig. 2(c) shows the FESEM image of MG-2. The morphologies of MG-1, MG-3, and MG-4 (see Fig. S1, ESI†) are similar to that of MG-2. It is clearly observed that the surface of curled RGO nanosheets is contacted with MoS2 well, which is beneficial for the separation of photo-generated carriers. The existence of MoS2 in the composite has been proved by EDS (Fig. 2(d)), and the ratio of Mo and S atoms is 1 to 2.14, which is close to the proportion of MoS2. | ||
| Fig. 2 Surface morphologies of (a) RGO nanosheets, (b) MoS2 and (c) MG-2 by FESEM measurements; (d) EDS spectrum of MG-2. | ||
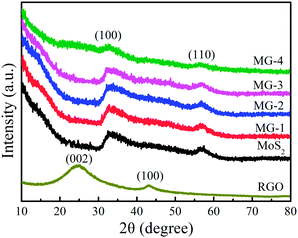
Fig. 3 shows the XRD patterns of RGO, MoS2 and MoS2–RGO composites. The RGO nanosheets exhibit a (002) diffraction peak at 26° and a (100) peak at 44.5°. The XRD analysis shows that the main diffraction peaks of MoS2–RGO composites are similar to those of pure MoS2 and correspond to those of hexagonal phase MoS2 (JCPDS 77-1716),42 which demonstrates that the presence of RGO does not result in the development of new crystal orientations of MoS2. No typical diffraction peaks of carbon species are observed in the composites when the content of RGO is less than 1 wt%, which may be due to the low amount of RGO. When the content of the RGO is 1 wt%, the RGO (002) peak appears in the XRD pattern of MG-4 though it is not so obvious.
The charge transfer and recombination behavior of the as-prepared samples was studied by analyzing the EIS spectra. Fig. 4 shows the typical Nyquist plots of MoS2, MG-1, MG-2, MG-3 and MG-4. The semicircle in the EIS spectra is due to the contribution from the charge transfer resistance (Rct) and constant phase element (CPE) at the photocatalyst/electrolyte interface. The inclined line, resulting from the Warburg impedance (ZW), corresponds to the ion diffusion process in the electrolyte. The corresponding equivalent circuit is shown in the inset of Fig. 4. It is clearly observed that the Rct decreases with the increase in RGO content, indicating that the introduction of RGO suppresses the charge recombination, which is beneficial to photocatalysis. The UV-vis absorption spectra of MoS2, MG-1, MG-2, MG-3 and MG-4 are shown in Fig. 5, which are similar to those reported by others.43–45 The result shows that along with the increase in RGO content, the absorption of the MoS2–RGO composites increases, which is beneficial to photocatalysis. However, when the RGO content is further increased (MG-3 and MG-4), the absorption decreases, which is similar to the report by Liu et al.46
 | ||
| Fig. 4 Nyquist plots of MoS2, MG-1, MG-2, MG-3 and MG-4. Inset is the corresponding equivalent circuit model. | ||
It is well known that the adsorption of dyes is an important step for photocatalysis.47–49 Upon reaching the adsorption equilibrium in the dark for 30 min, the MB adsorptivity of MoS2, MG-1, MG-2, MG-3 and MG-4 was measured. Fig. 6 shows the result of MB adsorption experiments. The normalized temporal concentration changes (B/B0) of MB during the adsorption process are proportional to the normalized maximum absorbance (A/A0), which can be derived from the change in the MB absorption profile during the adsorption process. It is found that the adsorption of MB by the photocatalysts increases with the increase in RGO content, indicating that the introduction of RGO improves the adsorptivity of the composite, which is beneficial to photocatalysis. This result is similar to the report by Zhang et al.50
 | ||
| Fig. 6 Bar plot showing the remaining MB in the solution after reaching the adsorption equilibrium in the dark by the MoS2, MG-1, MG-2, MG-3 and MG-4 in 30 min. | ||
Photocatalytic degradation of MB by MoS2, MG-1, MG-2, MG-3 and MG-4 was performed under visible light irradiation after reaching the adsorption–desorption equilibrium in the dark for 30 min. Fig. 7(a) shows the UV-vis absorption spectra of MB at the varied irradiation time using MG-2. It is observed that the UV-vis absorption peak of MB, related to its concentration in the solution, becomes weak with the increase in the irradiation time. Fig. 7(b) displays the time-dependent degradation rates of MB by MoS2, MG-1, MG-2, MG-3 and MG-4. The normalized temporal concentration changes (C/C0) of MB during the photocatalysis process are proportional to the normalized maximum absorbance (A/A0), which can be derived from the change in the MB absorption profile during the photocatalysis process. It is observed that MoS2–RGO composites exhibit better photocatalytic performance than pure MoS2. The photocatalytic performance of MoS2–RGO composite depends on the proportion of RGO in the composite. The removal rate of MB for pure MoS2 is 56%. When RGO is introduced into MoS2, the removal rate is increased to 95% for MG-1, and reaches a maximum value of 99% for MG-2. The enhancement of the photocatalytic performance should be ascribed to the reduction in electron–hole pair recombination due to the stepwise energy level structure in the composite and the increase in the light absorption with the presence of RGO.46,51–53 In addition, the enhanced adsorptivity of the composite for organic dye is an important factor for improving the photocatalysis.50,54 When the RGO content is further increased, the removal rate is decreased to 96% and 63% for MG-3 and MG-4, respectively. The reason may be that excessive RGO in the composite increases the opportunity for the collision of electrons and holes and promotes the recombination of the photo-generated electron–hole pairs.55 Moreover, the lower light absorption of MG-3 and MG-4 should also be responsible for their worse photocatalytic performance.
4. Conclusions
MoS2–RGO composites were successfully synthesized via microwave-assisted reduction of GO in MoS2 precursor solution using a microwave system and their photocatalytic performances were investigated. The experimental results indicate that (i) MoS2–RGO composites exhibit a better photocatalytic performance than pure MoS2, and their photocatalytic performances are dependent on the proportion of RGO in the composites; (ii) the MoS2RGO composite with 0.5 wt% RGO achieves a highest MB degradation rate of 99% in 60 min. (iii) The enhanced photocatalytic performance is ascribed to the increased light adsorption and adsorptivity for MB, as well as the reduction in photoelectron–hole pair recombination in MoS2 with the introduction of RGO.Acknowledgements
Financial support from China Postdoctoral Science Foundation funded project (no. 2013M540379) is gratefully acknowledged.Notes and references
- L. Lin, Y. Yang, L. Men, X. Wang, D. He, Y. Chai, B. Zhao, S. Ghoshroy and Q. Tang, Nanoscale, 2013, 5, 588–593 RSC.
- X. Bai, L. Wang, R. Zong, Y. Lv, Y. Sun and Y. Zhu, Langmuir, 2013, 29, 3097–3105 CrossRef CAS PubMed.
- W. Yao, B. Zhang, C. Huang, C. Ma, X. Song and Q. Xu, J. Mater. Chem., 2012, 22, 4050–4055 RSC.
- S. Higashimoto, Y. Tanaka, R. Ishikawa, S. Hasegawa, M. Azuma, H. Ohue and Y. Sakata, Catal. Sci. Technol., 2013, 3, 400–403 CAS.
- Y. Wang, Z. Wang, S. Muhammad and J. He, CrystEngComm, 2012, 14, 5065–5070 RSC.
- S. E. Stanca, R. Müller, M. Urban, A. Csaki, F. Froehlich, C. Krafft, J. Popp and W. Fritzsche, Catal. Sci. Technol., 2012, 2, 1472–1479 CAS.
- Z. Xu, I. Tabata, K. Hirogaki, K. Hisada, T. Wang, S. Wang and T. Hori, Catal. Sci. Technol., 2011, 1, 397–400 CAS.
- Z. Zheng, J. Zhao, Y. Yuan, H. Liu, D. Yang, S. Sarina, H. Zhang, E. R. Waclawika and H. Zhu, Chem. - Eur. J., 2013, 19, 5731–5741 CrossRef CAS PubMed.
- R. Huang, H. Ge, X. Lin, Y. Guo, R. Yuan, X. Fu and Z. Li, RSC Adv., 2013, 3, 1235–1242 RSC.
- X. J. Liu, L. K. Pan, T. Lv, Z. Sun and C. Q. Sun, J. Colloid Interface Sci., 2013, 408, 145–150 CrossRef CAS PubMed.
- X. J. Liu, L. K. Pan, T. Lv, T. Lu, G. Zhu, Z. Sun and C. Q. Sun, Catal. Sci. Technol., 2011, 1, 1189–1193 CAS.
- T. Kamegawa, S. Matsuura, H. Seto and H. Yamashita, Angew. Chem., Int. Ed., 2013, 52, 916–919 CrossRef CAS PubMed.
- D. Lin, H. Wu, R. Zhang and W. Pan, Chem. Mater., 2009, 21, 3479–3484 CrossRef CAS.
- K. Chang and W. Chen, Chem. Commun., 2011, 47, 4252–4254 RSC.
- H. S. S. Ramakrishna Matte, A. Gomathi, A. K. Manna, D. J. Late, R. Datta, S. K. Pati and C. N. R. Rao, Angew. Chem., Int. Ed., 2010, 122, 4153–4156 CrossRef.
- K. H. Hu, Z. Liu, F. Huang, X. G. Hu and C. L. Han, Chem. Eng. J., 2010, 162, 836–843 CrossRef CAS PubMed.
- F. A. Frame and F. E. Osterloh, J. Phys. Chem. C, 2010, 114, 10628–10633 CAS.
- L. A. King, W. Zhao, M. Chhowalla, D. J. Riley and G. Eda, J. Mater. Chem. A, 2013, 1, 8935–8941 CAS.
- J. Brivio, D. T. L. Alexander and A. Kis, Nano Lett., 2011, 11, 5148–5153 CrossRef CAS PubMed.
- Y. Li, Y. Li, C. M. Araujo, W. Luo and R. Ahuja, Catal. Sci. Technol., 2013, 3, 2214–2220 CAS.
- B. Visic, R. Dominko, M. K. Gunde, N. Hauptman, S. D. Skapin and M. Remskar, Nanoscale Res. Lett., 2011, 6, 1–6 CrossRef PubMed.
- S. Min and G. Lu, J. Phys. Chem. C, 2012, 116, 25415–25424 CAS.
- D. James and T. Zubkov, J. Photochem. Photobiol., A, 2013, 262, 45–51 CrossRef CAS PubMed.
- K. H. Hu, X. G. Hu, Y. F. Xu and X. Z. Pan, React. Kinet., Mech. Catal., 2010, 100, 153–163 CAS.
- G. Eda, H. Yamaguchi, D. Voiry, T. Fujita, M. Chen and M. Chhowalla, Nano Lett., 2011, 11, 5111–5116 CrossRef CAS PubMed.
- Y. Chen, H. Ge, L. Wei, Z. Li, R. Yuan, P. Liu and X. Fu, Catal. Sci. Technol., 2013, 3, 1712–1717 CAS.
- N. Zhang, Y. Zhang, X. Pan, X. Fu, S. Liu and Y. J. Xu, J. Phys. Chem. C, 2011, 115, 23501–23511 CAS.
- L. K. Pan, X. J. Liu, Z. Sun and C. Q. Sun, J. Mater. Chem. A, 2013, 1, 8299–8326 CAS.
- S. Liu, J. Tian, L. Wang, Y. Luo and X. Sun, Catal. Sci. Technol., 2012, 2, 339–344 CAS.
- X. J. Liu, L. K. Pan, T. Lv and Z. Sun, J. Colloid Interface Sci., 2013, 394, 441–444 CrossRef CAS PubMed.
- J. Shen, B. Yan, M. Shi, H. Ma, N. Li and M. Ye, J. Mater. Chem., 2011, 21, 3415–3421 RSC.
- G. Williams, B. Seger and P. V. Kamat, ACS Nano, 2008, 2, 1487–1491 CrossRef CAS PubMed.
- T. Xu, L. Zhang, H. Cheng and Y. Zhu, Appl. Catal., B, 2011, 101, 382–387 CrossRef CAS PubMed.
- Q. Xiang, J. Yu and M. Jaroniec, Nanoscale, 2011, 3, 3670–3678 RSC.
- Z. Xiong, L. Zhang, J. Ma and X. S. Zhao, Chem. Commun., 2010, 46, 6099–6101 RSC.
- G. Jiang, Z. Lin, C. Chen, L. Zhu, Q. Chang, N. Wang, W. Wei and H. Tang, Carbon, 2011, 49, 2693–2701 CrossRef CAS PubMed.
- T. Lv, L. K. Pan, X. J. Liu and Z. Sun, Electrochim. Acta, 2012, 83, 216–220 CrossRef CAS PubMed.
- H. B. Li, T. Lu, L. K. Pan, Y. P. Zhang and Z. Sun, J. Mater. Chem., 2009, 19, 6773–6779 RSC.
- T. Lu, Y. P. Zhang, H. B. Li, L. K. Pan, Y. L. Li and Z. Sun, Electrochim. Acta, 2010, 55, 4170–4173 CrossRef CAS PubMed.
- X. J. Liu, L. K. Pan, T. Lv, G. Zhu, Z. Sun and C. Q. Sun, Chem. Commun., 2011, 47, 11984–11986 RSC.
- T. Lv, L. Pan, X. Liu, T. Lu, G. Zhu and Z. Sun, J. Alloys Compd., 2011, 509, 10086–10091 CrossRef CAS PubMed.
- Y. Li, H. Wang, L. Xie, Y. Liang, G. Hong and H. Dai, J. Am. Chem. Soc., 2011, 133, 7296–7299 CrossRef CAS PubMed.
- A. B. Laursen, T. Pedersen, P. Malacrida, B. Seger, O. Hansen, P. C. Vesborg and I. Chorkendorff, Phys. Chem. Chem. Phys., 2013, 15, 20000–20004 RSC.
- Y. Yao, Z. Lin, Z. Li, X. Song, K.-S. Moon and C.-p. Wong, J. Mater. Chem., 2012, 22, 13494–13499 RSC.
- X. Zong, Y. Na, F. Wen, G. Ma, J. Yang, D. Wang, Y. Ma, M. Wang, L. Sun and C. Li, Chem. Commun., 2009, 4536–4538 RSC.
- B. Liu, Y. Huang, Y. Wen, L. Du, W. Zeng, Y. Shi, F. Zhang, G. Zhu, X. Xu and Y. Wang, J. Mater. Chem., 2012, 22, 7484–7491 RSC.
- H. Zhang, X. Lv, Y. Li, Y. Wang and J. Li, ACS Nano, 2009, 4, 380–386 CrossRef PubMed.
- W. Zhou, Z. Yin, Y. Du, X. Huang, Z. Zeng, Z. Fan, H. Liu, J. Wang and H. Zhang, Small, 2013, 9, 140–147 CrossRef CAS PubMed.
- S. J. Yang, J. H. Im, T. Kim, K. Lee and C. R. Park, J. Hazard. Mater., 2011, 186, 376–382 CrossRef CAS PubMed.
- Y. Zhang, Z. R. Tang, X. Fu and Y. J. Xu, ACS Nano, 2010, 4, 7303–7314 CrossRef CAS PubMed.
- X. An, C. Y. Jimmy, Y. Wang, Y. Hu, X. Yu and G. Zhang, J. Mater. Chem., 2012, 22, 8525–8531 RSC.
- Q. Xiang, J. Yu and M. Jaroniec, Chem. Soc. Rev., 2012, 41, 782–796 RSC.
- Y. Zhang, Y. Zhu, J. Yu, D. Yang, T. W. Ng, P. K. Wong and J. Yu, Nanoscale, 2013, 5, 6307–6310 RSC.
- Y. Fu, H. Chen, X. Sun and X. Wang, Appl. Catal., B, 2012, 111, 280–287 CrossRef PubMed.
- X.-Y. Zhang, H.-P. Li, X.-L. Cui and Y.-H. Lin, J. Mater. Chem., 2010, 20, 2801–2806 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra46956e |
| This journal is © The Royal Society of Chemistry 2014 |