Tunable photoluminescence of europium-doped layered double hydroxides intercalated by coumarin-3-carboxylate†
Abstract
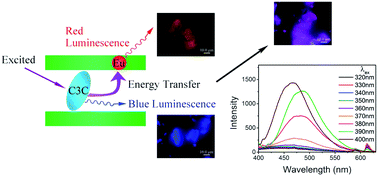
We report a highly tunable photoluminescence of rare-earth (europium, Eu3+) doped layered double hydroxides (LDHs) intercalated by an organic photofunctional anion, courmarin-3-carboxylate (C3C). Novel Eu3+-doped LDHs intercalated by C3C (ZnAlEu–LDH–C3C) with high purity and crystallinity have been successfully synthesized via an interlayer ion exchange process using Eu3+ doped LDH (ZnAlEu–LDH–NO3) as a precursor. A bilayer arrangement with nearly perpendicular orientation was deduced for the C3C anions in the LDH interlayer space. Compared with the precursor, ZnAlEu–LDH–C3C shows a strong UV absorbance and an interesting photoluminescence function, possibly due to an interfacial energy transfer process between the interlayer C3C anions and the Eu3+ within the LDH lattices. The photoluminescent spectra denote a lower symmetry of the coordinating environment around Eu3+ within ZnAlEu–LDH–C3C. More importantly, the photoluminescence of ZnAlEu–LDH–C3C could be highly tuned by simply adjusting the structural constituents (including the content of Eu3+ or C3C) or the excited wavelength. These findings open new avenues to prepare tunable photoluminescent materials and further have potential applications for optical devices.

 Please wait while we load your content...
Please wait while we load your content...