Bioinspired superhydrophobic surfaces with directional Adhesion†
Abstract
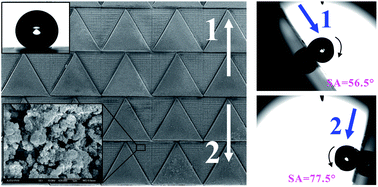
Butterfly wings have the ability to directionally control the movement of water microdroplets. However, the realization of artificial directional sliding biosurfaces has remained challenging. Inspired by butterfly wings, a new kind of directional patterned surface is developed to achieve superhydrophobicity and anisotropic adhesive properties at the one-dimensional level. The surface is composed of a hydrophobic triangle array and surrounding superhydrophobic structure. On the as-prepared surface, a droplet rolls along one direction distinctly easier than its opposite direction. The maximum anisotropy of sliding angles along two opposite directions can reach 21°. This unique ability is ascribed to the direction-dependent arrangement of the two-dimensional (2D) triangle array patterns. The directional adhesive superhydrophobic surfaces could be potentially applied in novel microfluid-controllable devices and directional easy-cleaning coatings.

 Please wait while we load your content...
Please wait while we load your content...