Novel oxovanadium(iv) complexes with 4-acyl pyrazolone ligands: synthesis, crystal structure and catalytic activity towards the oxidation of benzylic alcohols†
Abstract
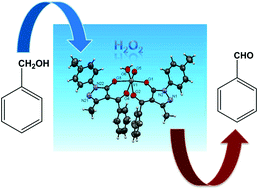
A series of oxovanadium(IV) complexes of 4-acylpyrazolone ligands were synthesized and characterized by elemental analyses, FT-IR, UV-Vis, EPR spectroscopy and single crystal XRD. The single-crystal X-ray analysis of the complex VO(L1)2 shows that the ligands were coordinated with the vanadium atom in a twisted form to create a distorted octahedral geometry and two O,O-chelating acylpyrazolonate ligands constitute two six-membered rings with the vanadium atom. The catalytic activity of all the complexes was evaluated for the oxidation of benzylic alcohols with H2O2 as an oxidant. The conditions for maximum conversion as well as selectivity for the desired product were optimized by varying different parameters such as the molar ratio of substrate to H2O2, the amount of the catalyst, reaction time and solvent. A possible pathway for the oxidation of benzylic alcohols was also proposed on the basis of the spectral evidence.

 Please wait while we load your content...
Please wait while we load your content...