Enhancement and wettability of self-assembled GO sheets as interfacial layers of CF/PI composites
Abstract
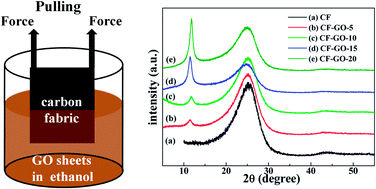
The self-assembled GO interlayers are easily fabricated by a dip coating process. Carbon fiber reinforced polyimide (CF/PI) composites are prepared by the infiltration process and compression moulding. The GO interfacial properties with different cycles are investigated by scanning electron microscopy (SEM), X-ray diffraction (XRD) and static contact angle measurement. Furthermore, the mechanical properties are measured by the three point flexural test. And the fracture modes are determined by the SEM morphologies of the fracture surfaces.

 Please wait while we load your content...
Please wait while we load your content...