Polymer synthesis inside a nanospace of a surfactant–micelle on carbon nanotubes: creation of highly-stable individual nanotubes/ultrathin cross-linked polymer hybrids†
Yusuke Tsutsumia,
Tsuyohiko Fujigaya*ab and
Naotoshi Nakashima*abc
aDepartment of Applied Chemistry, Graduate School of Engineering, Kyushu University, 744 Motooka, Fukuoka, Japan. E-mail: nakashima-tcm@mail.cstm.kyushu-u.ac.jp; fujigaya-tcm@mail.cstm.kyushu-u.ac.jp; Fax: +81-92-802-2840
bInternational Institute for Carbon-Neutral Energy Research (WPI-I2CNER), Kyushu University, Japan
cJapan Science and Technology Agency (JST), Core Research of Evolutional Science & Technology (CREST), 5 Sanbancho, Chiyoda-ku, Tokyo 102-0075, Japan
First published on 23rd December 2013
Abstract
We describe a novel synthetic method towards single-walled carbon nanotubes (SWNTs)/polymer hybrids utilizing the nanospace of a surfactant–micelle on the SWNTs, which provides highly stable SWNTs/ultrathin cross-linked polymer networks. In this study, N-isopropylacrylamide (NIPAM) is used as the monomer for the SWNT-wrapping. The prepared SWNTs wrapped by the poly(NIPAM) are stable even after freeze-drying/redispersion cycles as well as after the addition of a large excess of surfactant molecules, and the stability is much higher than that of previously reported phospholipid-wrapped SWNTs that have been often used for the bio-applications of SWNTs. The present method is simple and opens a way to design and fabricate stable individually dissolved SWNT/polymer hybrids that are useful for many applications including bio-applications since a variety of (functional) monomers are readily applicable to the present method.
Introduction
Functionalization of carbon nanotubes (CNTs) is of importance for fundamental studies and the applications of CNTs since as-produced CNTs form aggregated/bundle structures and their dispersion in solution requires CNT functionalization.1–3 Especially, biological applications, one of the most promising applications for CNTs, strongly require a stable functionalization since the aggregation of CNTs has the risk of toxicity in vivo.4,5 As a functionalization method, chemical functionalization based on the covalent bond provides a stable modification of the CNT surfaces.6,7 However, the introduction of the structural defects decreases the unique CNT features such as large area-hydrophobicity surfaces useful for hydrophobic drug loading on the surfaces.8 On the other hand, physical (noncovalent) modification based on the physical adsorption of the functional molecules onto the CNT surfaces is able to retain the structure and intrinsic properties of the CNTs. For CNT biological applications, we need to recognize that the physical adsorption of small molecules is a dynamic process and the exchange of the molecules on the CNTs with the molecules in the bulk solution is always occurring,9 which causes the removal of the adsorbed molecules from the CNT surfaces inside the body. The use of a polymeric dispersant dramatically improves the stability of the CNTs through a multipoint physical adsorption,10 but it has been revealed that the wrapped polymer is unwrapped when a large number of molecules having a strong affinity with the CNTs are added.11 Indeed, Cherukuri et al. reported that the injection of polymer-wrapped single-walled carbon nanotubes (SWNTs) into blood sera resulted in rapid displacement by endogenous proteins since some proteins very strongly adsorb onto the SWNT surface.12 Such a risk of aggregation and the replacement in vivo requires a new functionalization method to enhance the dispersion stability.In 2003, Taton et al. reported a pioneering study to stabilize polymer-wrapped SWNTs based on the cross-linking of the polymers around the CNTs.13 Similarly, the cross-linking of surfactant micelles has been studied to improve the stability of the CNT dispersion states.14–17 Such cross-linking strategies provide a promising approach to achieve a highly stable and non-destructive CNT coating because the wrapping layers are stabilized based on the covalent bonding of polymers around the CNT surfaces. However, this approach has only been applicable to molecules that are able to disperse the CNTs, which prevents the versatility of the concept.
Here, we demonstrate a new strategy to synthesize a cross-linked polymer network with an ultrathin layer (∼nm-size) around the surface of the CNTs using the inner nanospace of a surfactant micelle as the regulated polymerization site (Fig. 1). It has been recognized that in the CNT solutions dispersed by surfactant micelles, the CNTs are encapsulated by the surfactant micelles to provide a stable dispersion of the CNTs.18,19 Recently, the molecular penetration inside the micelles due to the hydrophobicity of the interior space was reported.20–22 Therefore, the monomer or the polymer together with the cross-linker is expected to penetrate inside the interior of the micelles due to the relatively hydrophobic nature of the space and form a cross-linked polymer network around the CNTs. One of great advantages of our strategy is that there is no need for the monomers to disperse the CNTs since the CNT dispersion is carried out by the surfactant micelles, thus a variety of monomers are applicable to the present system.
Experimental section
Materials
Single-walled carbon nanotubes (SWNTs) were purchased from Carbon Nanotechnologies, Inc., and used as received. N-Isopropylacrylamide (NIPAM), N,N′-methylenebisacrylamide (BIS) and ammonium persulfate (APS) were purchased from Wako Pure Chemical Industries, Ltd. Sodium dodecyl sulfate (SDS) and poly(ethyleneglycol) methacrylate (PEG-MA) with the molecular weight = 526 were purchased from Sigma Aldrich. NIPAM was purified by recrystallization from n-hexane. All other reagents were used without further purification.Synthesis of SWNT/PNIPAM
The SWNTs (6 mg) were dispersed in a 0.2 wt% SDS aqueous solution (60 mL) by ultrasonication (Branson 5510) for 1 h, and then centrifuged at 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g (Hitachi himac, CS 150 GX) for 1 h. The top 80% of the supernatant solution was collected and sonicated for a further 30 min, to which (25 mL) NIPAM (130 mg) and BIS (10 mg) were added and solubilized in the solution, and then the mixture was bubbled with N2 gas for 1 h to remove any residual oxygen. After adding a 20-wt% aqueous solution of APS (29 μL) to the mixture, the polymerization was carried out at 70 °C for 7 h under a nitrogen atmosphere. After the removal of a small amount of generated precipitate by filtration using a cotton filter, the obtained solution was then filtered 7 times using a membrane filter (molecular weight cut-off: MWCO = 10
000g (Hitachi himac, CS 150 GX) for 1 h. The top 80% of the supernatant solution was collected and sonicated for a further 30 min, to which (25 mL) NIPAM (130 mg) and BIS (10 mg) were added and solubilized in the solution, and then the mixture was bubbled with N2 gas for 1 h to remove any residual oxygen. After adding a 20-wt% aqueous solution of APS (29 μL) to the mixture, the polymerization was carried out at 70 °C for 7 h under a nitrogen atmosphere. After the removal of a small amount of generated precipitate by filtration using a cotton filter, the obtained solution was then filtered 7 times using a membrane filter (molecular weight cut-off: MWCO = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) to remove the large excess amounts of SDS molecules and the unreacted monomers. SWNT/PNIPAM-PEG was synthesized by a similar polymerization protocol in the presence of PEG-MA as the co-monomer.
000) to remove the large excess amounts of SDS molecules and the unreacted monomers. SWNT/PNIPAM-PEG was synthesized by a similar polymerization protocol in the presence of PEG-MA as the co-monomer.
Measurements
The UV-vis-NIR spectra were measured at specified temperatures by a spectrophotometer (JASCO, V-670). The photoluminescence (PL) spectra were measured using a spectrofluorometer (Horiba JOVIN YVON, FL3-21) with the integration time of 5 s. The 10 nm slit width and 4 nm scan steps were selected for both excitation and emission. Temperature-controlled dynamic light scattering (DLS) measurements were conducted using a particle size analysis system (Otsuka Electronics, ELSZ-2). An atomic force microscope (AFM) study was performed using a probe microscope (Agilent, 5500) with a SiN probe having the spring constant of 10–130 N m−1 in the tapping mode. The 1H NMR and X-ray photoelectron spectroscopy (XPS) measurements were carried out using an AV300 M spectrometer (BRUKER BIOSPIN) and AXIS-ULTRA DLD (SHIMADZU), respectively.Results and discussion
Preparation of SWNT coated with cross-linked PNIPAM
SWNTs dispersed in an aqueous SDS micelle were added to N-isopropyl acrylamide (NIPAM), ammonium persulfate (APS) and N,N′-methylenebisacrylamide (BIS) as the monomer, initiator and cross-linker, respectively, and the polymerization was carried out at 70 °C for 7 h. After removing a small amount of aggregation by filtration through cotton, the obtained solution was filtered to remove the SDS together with the unreacted reagents using a filter (MWCO; 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000). The removal of the SDS was confirmed by an X-ray photoelectron spectroscopy (XPS) measurement from which the residual peaks of the sulfur atom were not detected (see ESI, Fig. S1†). Fig. 2a shows a photograph after the dispersion of the filtered SWNTs, in which a transparent and black-colored solution without any visible aggregation was obtained. On the contrary, the control samples prepared in the absence of either the initiator (Fig. 2b) or cross-linker (Fig. 2c) provided aggregates, indicating that the cross-linking of the polymer serves an essential role for the stable dispersion in water.
000). The removal of the SDS was confirmed by an X-ray photoelectron spectroscopy (XPS) measurement from which the residual peaks of the sulfur atom were not detected (see ESI, Fig. S1†). Fig. 2a shows a photograph after the dispersion of the filtered SWNTs, in which a transparent and black-colored solution without any visible aggregation was obtained. On the contrary, the control samples prepared in the absence of either the initiator (Fig. 2b) or cross-linker (Fig. 2c) provided aggregates, indicating that the cross-linking of the polymer serves an essential role for the stable dispersion in water.
Structural analyses of the sample were carried out using an atomic force microscope (AFM) and dynamic light scattering (DLS). In the AFM, fibrous and needle-like structures that would be composed of the SWNTs coated with the cross-linked PNIPAM (SWNT/PNIPAM) (marked by the arrows) together with the particle structures (marked by the circle) were observed (Fig. 3a). The presence of two types of structures was also provided by the DLS study shown in Fig. 3b in which we observe bimodal peaks at around ∼25 nm and ∼210 nm. Since the polymerization could take place not only inside the surfactant micelles containing the SWNTs but also in the micelles without containing the SWNTs, the round-shaped particles were considered to be formed under the micelle not containing the SWNT,23–25 which provided round-shaped structures in the AFM image and a small-size distribution in the DLS.
 | ||
| Fig. 3 (a and c) AFM images (5 × 5 μm) and (b and d) DLS histograms of SWNT/PNIPAM in an aqueous solution after the (a and b) filtration and (c and d) centrifugation. | ||
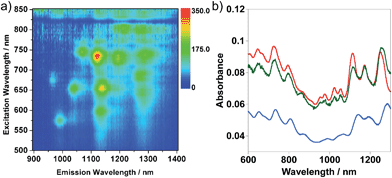
Quite interestingly, we discovered that centrifugation of the solution containing two different structures at 120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g preferentially flocculated SWNT/PNIPAM with leaving the particle-like structures in the supernatant solution. Fig. 3c and d show the AFM image and DLS histogram of the sediment measured after redispersion in water. In the AFM image, it is evident that the most dominant shape was the needle-like structures (Fig. 3c). Based on the AFM measurement, the average diameter of SWNT/PNIPAM was found to be 3.05 nm (N = 59). Considering that the average SWNT diameter in this study is ∼1 nm, the thickness of the cross-linked PNIPAM was determined to be ∼1 nm. Furthermore, the intensity around 25 nm in the DLS almost disappeared while leaving that at ∼210 nm as a unimodal peak (Fig. 3d), while the intensity around 25 nm became more intense in the supernatant solution after the centrifugation (see ESI, Fig. S2†). We assumed that the SWNT/PNIPAM was preferentially sediment by the ultracentrifugation probably due to the higher density of the structure by the encapsulation of the SWNTs. The presence of an isolated SWNT inside SWNT/PNIPAM was revealed by the PL signals emitted from the SWNTs as shown in Fig. 4a since the PL was observed only when the SWNTs are isolated.26
000g preferentially flocculated SWNT/PNIPAM with leaving the particle-like structures in the supernatant solution. Fig. 3c and d show the AFM image and DLS histogram of the sediment measured after redispersion in water. In the AFM image, it is evident that the most dominant shape was the needle-like structures (Fig. 3c). Based on the AFM measurement, the average diameter of SWNT/PNIPAM was found to be 3.05 nm (N = 59). Considering that the average SWNT diameter in this study is ∼1 nm, the thickness of the cross-linked PNIPAM was determined to be ∼1 nm. Furthermore, the intensity around 25 nm in the DLS almost disappeared while leaving that at ∼210 nm as a unimodal peak (Fig. 3d), while the intensity around 25 nm became more intense in the supernatant solution after the centrifugation (see ESI, Fig. S2†). We assumed that the SWNT/PNIPAM was preferentially sediment by the ultracentrifugation probably due to the higher density of the structure by the encapsulation of the SWNTs. The presence of an isolated SWNT inside SWNT/PNIPAM was revealed by the PL signals emitted from the SWNTs as shown in Fig. 4a since the PL was observed only when the SWNTs are isolated.26
Fig. 4b shows the absorption spectra of SWNT/PNIPAM at room temperature after the separation by ultracentifugation (blue line). The absorption spectra of the as-prepared SWNTs dissolved in an aqueous SDS micelle before (red line in Fig. 4b) and after (green line in Fig. 4b) the addition of the monomer and the cross-linker are also displayed for comparison. Based on the decrease in the absorbance after the separation, the overall SWNTs yield was estimated to be ∼60% compared to that before the separation. Clear absorption peaks of the isolated SWNTs in the near-IR region (1100–1300 nm) due to the semiconducting SWNTs (S11 bands) allowed us to monitor the microenvironment around the SWNTs.27 Compared to the SWNT absorption spectra in the SDS micelle, after the PNIPAM coating, the peaks were shifted by ∼25 nm to a longer wavelength. Since the addition of the monomer caused no such shift, it is after the polymerization that the microenvironment around the SWNTs was changed.
Considering the phase transition temperature of PNIPAM (∼32 °C) in water,28,29 we propose a possible mechanism for the coating similar to the mechanism proposed for the emulsion polymerization of PNIPAM30 (Fig. 5); namely, (i) phase transition of NIPAM oligomers from hydrophilic to hydrophobic at 70 °C, and then (ii) penetration of the hydrophobic NIPAM oligomers together with a cross-linker into the SDS micelle to form a PNIPAM network structure around the SWNTs.
 | ||
| Fig. 5 Schematic model of the formation of a cross-linked network around SWNT using the interior of a surfactant micelle as the reaction site. | ||
Incorporation of additional functional groups in the PNIPAM network structure
In our method, we can easily introduce additional functional groups in the PNIPAM network structure on the SWNTs. In this study, as the co-monomer, poly(ethyleneglycol) methacrylate (PEG-MA) was selected to synthesize the biocompatible SWNTs. A similar synthesis and separation procedure were carried out in the presence of the PEG-MA at the feeding ratio of NIPAM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PEG-MA = 100
PEG-MA = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14. Based on NMR spectrum of the product, the copolymerization ratio was determined to be NIPAM
14. Based on NMR spectrum of the product, the copolymerization ratio was determined to be NIPAM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PEG-MA = 100
PEG-MA = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. We observed fibrous and needle-like structures in the AFM image (Fig. 6a), and found that the peak intensity in the DLS histogram at around 30 nm is weak compared to that of the supernatant solution after the centrifugation (see ESI, Fig. S3†). These results obviously indicated the successful preparation and separation of the SWNTs coated with the cross-linked PNIPAM/PEG-MA copolymer (SWNT/PNIPAM-PEG) (see also ESI, Fig. S4†).
10. We observed fibrous and needle-like structures in the AFM image (Fig. 6a), and found that the peak intensity in the DLS histogram at around 30 nm is weak compared to that of the supernatant solution after the centrifugation (see ESI, Fig. S3†). These results obviously indicated the successful preparation and separation of the SWNTs coated with the cross-linked PNIPAM/PEG-MA copolymer (SWNT/PNIPAM-PEG) (see also ESI, Fig. S4†).
 | ||
Fig. 6 (a) AFM image (5 × 5 μm) and (b) DLS histogram of the SWNT/PNIPAM-PEG after centrifugation at 120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g. 000g. | ||
Fig. 7 shows the temperature-dependent absorption spectra of the SWNT/PNIPAM-PEG together with the SWNT/PNIPAM. Upon heating, the SWNT/PNIPAM showed an increased absorbance above 45 °C due to light scattering derived from the small aggregates generated by the hydrophilic-to-hydrophobic transition of the PNIPAM. On the other hand, for the SWNT/PNIPAM-PEG, no such change was observed even after heating to 70 °C, indicating no phase transition in this temperature range. Considering the fact that the incorporation of the PEG-MA increases the transition temperature depending on the PEG-MA content,31 the phase transition of the SWNT/PNIPAM-PEG is expected to be higher than 70 °C. Such a dispersion stability at the higher temperatures is essential especially for bio-applications, e.g. thermotherapy in vivo.
Coating stability of PNIPAM-PEG network structure
The coating stability around the CNTs is often tested by a freeze-drying and redispersion cycle.14,17 The dispersion of the freeze-dried SWNT/PNIPAM-PEG powder (Fig. 8a) in water by simple sonication for 2 min immediately provided a black-colored transparent solution without causing any aggregation (Fig. 8b), and no peak shift in the absorption spectra of the sample after the re-dispersion was observed as shown in Fig. 8c.It is true that such a stable dispersion after the freeze-drying has been recognized not only in the cross-linked system, but also in the tightly-adsorbed polymeric dispersant such as DNA-wrapped SWNTs,32 suggesting that the freeze-drying test is not sufficient to guarantee the high stability of the coating. It has been reported that the addition of an excess amount of a surfactant induces the detachment of the tightly-adsorbed DNA molecules from the SWNT surfaces.33 To further explore the coating stability, a large excess of SDS was added to examine whether or not it induces the removal of the wrapped polymer. As the control, SWNTs dispersed with a phospholipid linked with PEG (PL-PEG) was also tested since PL-PEG has been extensively studied as a promising candidate of the SWNT dispersant for biological applications due to their high dispersion stability in vivo.8,34–36 Fig. 9a and b show photographs after the addition of SDS followed by five filtrations to remove the SDS using a membrane filter (MWCO; 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000). As a result, aggregation of the SWNTs was observed for the SWNTs dispersed with the PL-PEG (SWNT/PL-PEG) after the several cycles. In sharp contrast, the SWNT/PNIPAM-PEG retained a stable dispersion during the filtration and dispersion cycles, and no such aggregates were recognized. The generation of aggregates for SWNT/PL-PEG is probably due to the removal of PL-PEG from the SWNT surfaces triggered by the addition of SDS, while, for SWNT/PNIPAM-PEG, the cross-linked PNIPAM network stably remained around the SWNT surface even after the addition of the large excess of SDS followed by washing as shown in Fig. 9d (bottom).
000). As a result, aggregation of the SWNTs was observed for the SWNTs dispersed with the PL-PEG (SWNT/PL-PEG) after the several cycles. In sharp contrast, the SWNT/PNIPAM-PEG retained a stable dispersion during the filtration and dispersion cycles, and no such aggregates were recognized. The generation of aggregates for SWNT/PL-PEG is probably due to the removal of PL-PEG from the SWNT surfaces triggered by the addition of SDS, while, for SWNT/PNIPAM-PEG, the cross-linked PNIPAM network stably remained around the SWNT surface even after the addition of the large excess of SDS followed by washing as shown in Fig. 9d (bottom).
The process was successfully monitored by absorption spectroscopy as shown in Fig. 9c. The absorption peaks of the SWNT/PNIPAM-PEG shifted to a shorter wavelength by 7–8 nm after the addition of SDS, corresponding to the change in the micro-dielectric environment through the incorporation of SDS molecules into the cross-linked polymer network layer (Fig. 9d; lower). After the removal of SDS by washing, the shifted peaks were returned to the original position (black line in Fig. 9c), which indicates the detachment of SDS from the SWNT surface and the restoration of the initial state.
As other aspect, the above results suggest that the function of the coated SWNTs as a molecular container enabling the encapsulating and releasing of the molecules without involving any destruction of the coating layer, which is attractive for potential drug delivery applications. Currently, since the NIPAM-based copolymerization has been reported for various monomers, such as monomer-carrying fluorescent dyes37–39 and cancer ligands,40,41 further functionalization of the wrapping layer is possible. We expect the SWNTs wrapped with the cross-linked PNIPAM prepared by the present strategy are attractive nanomaterials for many biological applications. In vivo studies using the material is now underway in our laboratory.
Conclusions
We described a simple method to wrap SWNTs with an ultrathin-cross-linked polymer network utilizing the nano-space between the surfactant layer and SWNT surfaces as the reaction site as schematically presented in Fig. 1. The cross-linked polymer-coated SWNTs showed a high stability upon heating, freeze-drying and the addition of a large amount of surfactant. Such a high stability enabled the adsorption and desorption of a small molecule without involving any destruction of the cross-linked network. The present method is simple and applicable to a wide variety of monomers to synthesize the designed functional CNTs wrapped by the polymer networks, which may lead to many potential applications in materials science and technology. Fluorescent labeling as well as ligand introduction into the networks on the CNTs are also of interest as materials for bio-applications. Such studies are currently underway in our laboratory.Acknowledgements
This work was supported in part by the Low-Carbon Research Network (LCnet) and the Nanotechnology Platform Project (Molecules and Materials Synthesis) of the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.Notes and references
- T. Fujigaya and N. Nakashima, Polym. J., 2008, 40, 577 CrossRef CAS.
- N. Nakashima and T. Fujigaya, Chem. Lett., 2007, 36, 692 CrossRef CAS.
- S. Banerjee, T. Hemraj-Benny and S. S. Wong, Adv. Mater., 2005, 17, 17 CrossRef CAS.
- X. Zhao and R. Liu, Environ. Int., 2012, 40, 244 CrossRef CAS PubMed.
- G. M. Mutlu, G. R. Budinger, A. A. Green, D. Urich, S. Soberanes, S. E. Chiarella, G. F. Alheid, D. R. McCrimmon, I. Szleifer and M. C. Hersam, Nano Lett., 2010, 10, 1664 CrossRef CAS PubMed.
- N. Karousis, N. Tagmatarchis and D. Tasis, Chem. Rev., 2010, 110, 5366 CrossRef CAS PubMed.
- D. Tasis, N. Tagmatarchis, A. Bianco and M. Prato, Chem. Rev., 2006, 106, 1105 CrossRef CAS PubMed.
- Z. Liu, A. C. Fan, K. Rakhra, S. Sherlock, A. Goodwin, X. Chen, Q. Yang, D. W. Felsher and H. Dai, Angew. Chem., Int. Ed., 2009, 48, 7668 CrossRef CAS PubMed.
- A. Ishibashi and N. Nakashima, Bull. Chem. Soc. Jpn., 2006, 79, 357 CrossRef CAS.
- Y. Noguchi, T. Fujigaya, Y. Niidome and N. Nakashima, Chem. Phys. Lett., 2008, 455, 249 CrossRef CAS PubMed.
- D. Roxbury, X. Tu, M. Zheng and A. Jagota, Langmuir, 2011, 27, 8282 CrossRef CAS PubMed.
- P. Cherukuri, C. J. Gannon, T. K. Leeuw, H. K. Schmidt, R. E. Smalley, S. A. Curley and R. B. Weisman, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 18882 CrossRef CAS PubMed.
- Y. Kang and T. A. Taton, J. Am. Chem. Soc., 2003, 125, 5650 CrossRef CAS PubMed.
- T. H. Kim, C. Doe, S. R. Kline and S. M. Choi, Adv. Mater., 2007, 19, 929 CrossRef CAS.
- C. Thauvin, S. Rickling, P. Schultz, H. Celia, S. Meunier and C. Mioskowski, Nat. Nanotechnol., 2008, 3, 743 CrossRef CAS PubMed.
- C. d. Thauvin, A. l. Perino, E. Contal, E. Morin, P. Schultz, S. p. Meunier and A. Wagner, J. Phys. Chem. C, 2011, 115, 7319 CAS.
- R. Wang, P. Cherukuri, J. G. Duque, T. K. Leeuw, M. K. Lackey, C. H. Moran, V. C. Moore, J. L. Conyers, R. E. Smalley, H. K. Schmidt, R. B. Weisman and P. S. Engel, Carbon, 2007, 45, 2388 CrossRef CAS PubMed.
- L. Vaisman, H. D. Wagner and G. Marom, Adv. Colloid Interface Sci., 2006, 128–130, 37 CrossRef CAS PubMed.
- P. Angelikopoulos and H. Bock, Phys. Chem. Chem. Phys., 2012, 14, 9546 RSC.
- C. Roquelet, J. S. Lauret, V. Alain-Rizzo, C. Voisin, R. Fleurier, M. Delarue, D. Garrot, A. Loiseau, P. Roussignol, J. A. Delaire and E. Deleporte, ChemPhysChem, 2010, 11, 1667 CrossRef CAS PubMed.
- R. K. Wang, W. C. Chen, D. K. Campos and K. J. Ziegler, J. Am. Chem. Soc., 2008, 130, 16330 CrossRef CAS PubMed.
- C. A. Silvera-Batista, R. K. Wang, P. Weinberg and K. J. Ziegler, Phys. Chem. Chem. Phys., 2010, 12, 6990 RSC.
- W. D. Harkins, J. Am. Chem. Soc., 1947, 69, 1428 CrossRef CAS.
- C. Y. Quan, H. Wei, Y. X. Sun, S. X. Cheng, K. Shen, Z. W. Gu, X. Z. Zhang and R. X. Zhuo, J. Nanosci. Nanotechnol., 2008, 8, 2377 CrossRef CAS PubMed.
- R. M. Ramanan, P. Chellamuthu, L. Tang and K. T. Nguyen, Biotechnol. Prog., 2006, 22, 118 CrossRef CAS PubMed.
- M. J. O'Connell, S. M. Bachilo, C. B. Huffman, V. C. Moore, M. S. Strano, E. H. Haroz, K. L. Rialon, P. J. Boul, W. H. Noon, C. Kittrell, J. Ma, R. H. Hauge, R. B. Weisman and R. E. Smalley, Science, 2002, 297, 593 CrossRef CAS PubMed.
- E. S. Jeng, A. E. Moll, A. C. Roy, J. B. Gastala and M. S. Strano, Nano Lett., 2006, 6, 371 CrossRef CAS PubMed.
- H. Ahmad, M. A. Rahman, M. A. J. Miah and K. Tauer, Macromol. Res., 2008, 16, 637 CrossRef CAS.
- S. R. Sershen, S. L. Westcott, N. J. Halas and J. L. West, J. Biomed. Mater. Res., 2000, 51, 293 CrossRef CAS.
- X. Wu, R. H. Pelton, A. E. Hamielec, D. R. Woods and W. McPhee, Colloid Polym. Sci., 1994, 272, 467 CAS.
- N. Gulati, R. Rastogi, A. K. Dinda, R. Saxena and V. Koul, Colloids Surf., B, 2010, 79, 164 CrossRef CAS PubMed.
- Y. Noguchi, Master's thesis, Kyushu University, 2008.
- Y. Kato, A. Inoue, Y. Niidome and N. Nakashima, Sci. Rep., 2012, 2, 733 Search PubMed.
- Z. Liu, X. Sun, N. Nakayama-Ratchford and H. Dai, ACS Nano, 2007, 1, 50 CrossRef CAS PubMed.
- H. K. Moon, S. H. Lee and H. C. Choi, ACS Nano, 2009, 3, 3707 CrossRef CAS PubMed.
- K. Welsher, S. P. Sherlock and H. J. Dai, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 8943 CrossRef CAS PubMed.
- Y. Matsumura and K. Iwai, Polymer, 2005, 46, 10027 CrossRef CAS PubMed.
- J. Yin, X. F. Guan, D. Wang and S. Y. Liu, Langmuir, 2009, 25, 11367 CrossRef CAS PubMed.
- L. Yin, C. He, C. Huang, W. Zhu, X. Wang, Y. Xu and X. Qian, Chem. Commun., 2012, 48, 4486 RSC.
- C. Y. Quan, D. Q. Wu, C. Chang, G. B. Zhang, S. X. Cheng, X. Z. Zhang and R. X. Zhuo, J. Phys. Chem. C, 2009, 113, 11262 CAS.
- J. Zhang, D. Deng, H. Zhu, Y. Byun, V. C. Yang and Y. Gu, J. Biomed. Mater. Res., Part A, 2012, 100, 3134 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: XPS scans of SWNT/PNIPAM, DLS histogram of a supernatant solution of SWNT/PNIPAM after the centrifugation, and PL mapping and absorption spectrum of an aqueous solution of SWNT/PNIPAM-PEG. See DOI: 10.1039/c3ra46841k |
| This journal is © The Royal Society of Chemistry 2014 |