Self-assembled synthesis of carbon-coated Fe3O4 composites with firecracker-like structures from catalytic pyrolysis of polyamide
Abstract
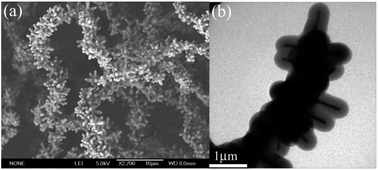
Carbon-coated Fe3O4 composites with firecracker-like structures have been fabricated by catalytic pyrolysis of polyamide (PA) in a sealed reaction system. As revealed in field-emission scanning electron microscopy (FESEM) and transmission electron microscopy (TEM) analysis, the diameter of the one-dimensional firecracker-like structures is about 4 μm and the diameter of the secondary nanorods is about 620 nm. The diameter of Fe3O4 nanorods inside the carbon shells is about 82 nm, and the thickness of the carbon shells is about 265 nm. Some important preparative parameters related to the synthesis have been identified and investigated by some designed experiments. The magnetic measurement at room temperature indicates that the values of saturation magnetization (19.2 emu g−1) and coercivity (270.1 Oe) of the carbon-coated Fe3O4 composites with firecracker-like structures are different from those of Fe3O4 nanoparticles and bulk Fe3O4 due to the different carbon content, dipolar interaction, size and morphology of the products. The results also indicate that the one-dimensional Fe3O4@C core–shell structures possess good acid resistance.

 Please wait while we load your content...
Please wait while we load your content...