Mechanical and metallic properties of tantalum nitrides from first-principles calculations
Da Li,
Fubo Tian,
Defang Duan,
Kuo Bao,
Binhua Chu,
Xiaojing Sha,
Bingbing Liu and
Tian Cui*
State Key Lab of Superhard Materials, College of Physics, Jilin University, Changchun 130012, P.R. China. E-mail: cuitian@jlu.edu.cn; Fax: +86-431-85168825; Tel: +86-431-85168825
First published on 28th January 2014
Abstract
The phase stability, mechanical properties and metallic properties of tantalum nitrides are extensively studied by means of first principles calculations. The relationship between nitrogen concentration and physical properties of tantalum nitrides has been systematically investigated. With the nitrogen concentration increasing, it is found that the feature of covalent bonding enhances and the directionality of the covalent bonding and hardness of tantalum nitrides reduce. While these make the ductility of tantalum nitrides improve with the nitrogen concentration increasing. The intensity of metallic properties of tantalum nitrides can be effectively adjusted by controlling the nitrogen concentration and pressure. When the tantalum: nitrogen ratio reaches Ta![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N = 1
N = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, remarkable nitrogen–nitrogen bonds are found in TaN3. The hardness of TaN3 abnormally increases with reference to that of the preceding composition Ta3N5-II. The potential synthesis routes of tantalum nitrides are suggested.
3, remarkable nitrogen–nitrogen bonds are found in TaN3. The hardness of TaN3 abnormally increases with reference to that of the preceding composition Ta3N5-II. The potential synthesis routes of tantalum nitrides are suggested.
1 Introduction
Superhard materials have attracted much attention due to their wide industrial and technological applications. Typical superhard materials come from nature. Diamond is the most important one in typical superhard materials.1 Recently, to satisfy complicated industrial requirements, some novel superhard materials with unique physical and chemical properties, such as c-BN,2 C3N4,3 BC2N,4 and BC5 (ref. 5) etc., have been designed and synthesized by using theoretical and experimental methods. Over the past few decades, it has been proposed that the intercalation of light elements into transition metals might be a good strategy in the search for potential superhard materials. Very recently, four noble metal nitrides PtN,6 PtN2,7 IrN2,8 and OsN2 (ref. 8) have been successfully synthesized by diamond anvil techniques under high pressure and high temperature (HPHT).The tantalum (Ta) has similar valence electron arrangement and bulk modulus to osmium (Os), iridium (Ir), and platinum (Pt) in the periodic table of elements. So tantalum nitrides are expected to have the same good mechanical properties as osmium, iridium, and platinum nitrides. And tantalum and nitrogen can form semiconductor or weak metallic compounds with different compositions. The tantalum nitrides display rich crystal chemistry. In the Ta–N binary phase diagram, tantalum nitrides have six experimentally known compositions: Ta2N, TaN, Ta5N6, Ta4N5, Ta3N5, and Ta2N3. The α-Ta2N is an amorphous phase of Ta2N.9 β-Ta2N adopts the Fe2N-type structure.10 The TaN exists in three forms: θ-TaN (WC-type structure), δ-TaN (NaCl-type structure), and ε-TaN (CoSn-type structure).11,12 The first principle calculations indicate that the presence of Ta vacancies can reduce the density of state (DOS) around the Fermi level and result in a metal-to-insulator transition in δ-TaN.13 The Ta5N6 (space group: P63/mcm) crystallizes with hexagonal symmetry. The Ta4N5 (space group: I4/m) persists on the NaCl-like structure, consisting of an ordered arrangement of Ta vacancies, also exhibits a notable low DOS at the Fermi level.13 The lower DOS at Fermi level is the key for the formation of superhard materials.14 The most nitrogen-rich tantalum nitride Ta3N5-I (space group: Cmcm) is a semiconductor with a band gap of 1.5 eV.15–17 The high-pressure polymorph Ta3N5-II (space group: Pnma) is a potential hard material with a high bulk modulus of 378 GPa at above 9 GPa.15 Very recently, an orthorhombic U2S3-type Ta2N3 (space group: Pbnm) was synthesized under HPHT conditions.18 It exhibits a high hardness and an extraordinary texture and can be quenched to ambient condition. However, the theoretical study reveals that the experimentally observed U2S3-type Ta2N3 actually is an oxygen-substituted orthorhombic structure. Oxygen plays a key role for the stabilization of the experimentally observed U2S3-type Ta2N3 at ambient pressure. The extended enthalpy calculations indicate that tetragonal Ta2N3 (space group: P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) m2) is energetically more favorable to the experimentally observed orthorhombic U2S3-type Ta2N3 at below 7 GPa.19 Among the above mentioned tantalum nitrides, δ-TaN is superconducting (Tc = 6.5 K),20 and exhibits the highest hardness of 30–32 GPa in the group of transition metal mononitrides.21 Ta3N5 has attracted much attention as a visible-light-driven photocatalyst for splitting water.22 However, the current study of tantalum nitrides is still limited to lower nitrogen concentration (the highest nitrogen content reach to x = 1.67 in TaNx). There is lack of reports on the nitrogen-rich tantalum nitrides and the relationship between physical properties and nitrogen composition. The crystal structures, mechanical properties and metallic properties of nitrogen-rich tantalum nitrides are still far from being clear. It is of fundamental interest to explore nitrogen-rich structures in tantalum nitrides. Moreover, the stability, synthesis routes, and the origin of hardness of tantalum nitrides are least studied.
m2) is energetically more favorable to the experimentally observed orthorhombic U2S3-type Ta2N3 at below 7 GPa.19 Among the above mentioned tantalum nitrides, δ-TaN is superconducting (Tc = 6.5 K),20 and exhibits the highest hardness of 30–32 GPa in the group of transition metal mononitrides.21 Ta3N5 has attracted much attention as a visible-light-driven photocatalyst for splitting water.22 However, the current study of tantalum nitrides is still limited to lower nitrogen concentration (the highest nitrogen content reach to x = 1.67 in TaNx). There is lack of reports on the nitrogen-rich tantalum nitrides and the relationship between physical properties and nitrogen composition. The crystal structures, mechanical properties and metallic properties of nitrogen-rich tantalum nitrides are still far from being clear. It is of fundamental interest to explore nitrogen-rich structures in tantalum nitrides. Moreover, the stability, synthesis routes, and the origin of hardness of tantalum nitrides are least studied.
In this study, we report a systematic computational study on the crystal structures, stability, mechanical properties, metallic properties and synthesis routes of tantalum nitrides (TaNx, x ≥ 1). The crystal structures of nitrogen-rich tantalum nitrides are explored by ab initio evolutionary crystal structure prediction USPEX method.23–25 The relationship between mechanical and metallic properties and nitrogen concentration are systematically studied. The potential synthesis routes of tantalum nitrides are suggested. We uncover a synthesizable new composition TaN3 with strong covalent nitrogen–nitrogen bonds chains along the crystallographic b axis. Our study could be extremely helpful for future experiments.
2 Computational details
In this study, the experimentally observed compositions of TaN, Ta5N6, Ta4N5, Ta2N3, and Ta3N5 were adopted for the calculation. For nitrogen-rich tantalum nitrides, since no experimental structure parameters were found to date, we used the evolutionary algorithm (USPEX code),23–25 designed to search for the structure possessing the lowest free energy at given P/T conditions, to predict the possible structures. The evolutionary variable-cell structure predictions were performed at 0, 10, 30, 50, and 80 GPa with systems containing one, two, three, and four formula units (f.u.) in the simulation cell. In contrast to the early calculations and experiments, the underlying structure relaxations were performed using density functional theory (DFT) within the generalized gradient approximation (GGA),26 as implemented in the Vienna ab initio simulation package (VASP).27,28 The electron interaction was described by means of the projector-augmented wave method.29 The 2s2 2p3 and 6s2 5d3 were treated as valence electrons for N and Ta, respectively. Convergence tests gave a kinetic energy cutoff as 900 eV for all phases. Monkhorst–Pack30 k point meshes with a grid of 0.035 Å−1 for Brillouin zone sampling were chosen to ensure the total energies converged to be better than 1 meV per f.u. Gamma-point-centered k-mesh were used for the hexagonal structures. The phonon spectra were calculated using the direct supercell method, performed by PHONON software.31 The elastic constants were calculated by strain-stress method, and the bulk modulus, shear modulus, Young's modulus, and Poisson's ratio were thus derived from the Voigt–Reuss–Hill approximation.32–34 The theoretical Vickers hardness was estimated by using the Chen's model.14 Formation enthalpy was calculated by the equation of ΔH = E(TaxNy)−xE(Ta)−yE(N), in which bcc phase of Ta and α-N2 were adopted for the calculations. The details of convergence tests have been described elsewhere.35–373 Results and discussion
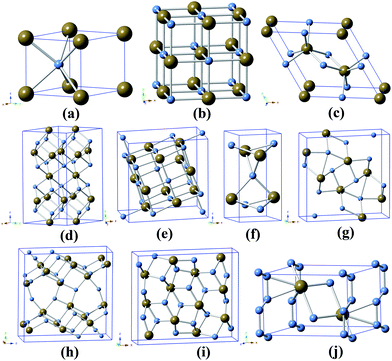
The optimized crystal structures of tantalum nitrides are shown in Fig. 1. Each Ta atom has six nearest neighboring N atoms in WC-type TaN, NaCl-type TaN, CoSn-type TaN, Ta5N6, and Ta4N5 structures. Each Ta atom has seven nearest neighboring N atoms in P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) m2-Ta2N3. The nearest neighbor number of Ta atoms is five or six in Ta3N5-I. The TaN3 has seven N atoms nearest neighboring Ta atom at 50 GPa. The calculated lattice parameters, cell volumes, and formation enthalpy of tantalum nitrides with various nitrogen concentrations in the paramagnetic and ferromagnetic states are summarized in Table 1. For tantalum mononitrides, the WC-type structure is energetically more stable than NaCl-type and CoSn-type structures at ambient pressure. The calculated lattice parameters of tantalum nitrides are in good agreement with the previous experimental results.11,38–40 So the parameters of the calculations used in the study are sufficient to predict Ta–N systems. The bond length of Ta–N bond is 2.235 Å in WC-type TaN, which is larger than the sum of bonding radii (2.09 Å) of Ta (r = 1.34 Å) and N (r = 0.75 Å) atoms. The Ta5N6 persists on the hexagonal structure with 22 atoms in the unit cell. The shortest bond length of Ta–N bond is 2.193 Å in Ta5N6, which is still larger than the sum of the bonding radii (2.09 Å), indicating the obvious weaker covalent bonding feature. The NaCl-like Ta4N5 has intrinsic Ta vacancies in the center of the structure as shown in Fig. 1. The shortest bond length of Ta–N bond is 2.086 Å in Ta4N5, which is very close to the sum of bonding radii (2.09 Å), indicating strong covalent bonding feature. The P
m2-Ta2N3. The nearest neighbor number of Ta atoms is five or six in Ta3N5-I. The TaN3 has seven N atoms nearest neighboring Ta atom at 50 GPa. The calculated lattice parameters, cell volumes, and formation enthalpy of tantalum nitrides with various nitrogen concentrations in the paramagnetic and ferromagnetic states are summarized in Table 1. For tantalum mononitrides, the WC-type structure is energetically more stable than NaCl-type and CoSn-type structures at ambient pressure. The calculated lattice parameters of tantalum nitrides are in good agreement with the previous experimental results.11,38–40 So the parameters of the calculations used in the study are sufficient to predict Ta–N systems. The bond length of Ta–N bond is 2.235 Å in WC-type TaN, which is larger than the sum of bonding radii (2.09 Å) of Ta (r = 1.34 Å) and N (r = 0.75 Å) atoms. The Ta5N6 persists on the hexagonal structure with 22 atoms in the unit cell. The shortest bond length of Ta–N bond is 2.193 Å in Ta5N6, which is still larger than the sum of the bonding radii (2.09 Å), indicating the obvious weaker covalent bonding feature. The NaCl-like Ta4N5 has intrinsic Ta vacancies in the center of the structure as shown in Fig. 1. The shortest bond length of Ta–N bond is 2.086 Å in Ta4N5, which is very close to the sum of bonding radii (2.09 Å), indicating strong covalent bonding feature. The P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) m2-Ta2N3 is energetically more favorable compared to the U2S3-type Ta2N3 at ambient pressure as summarized in Table 1. The shortest Ta–N bond length of P
m2-Ta2N3 is energetically more favorable compared to the U2S3-type Ta2N3 at ambient pressure as summarized in Table 1. The shortest Ta–N bond length of P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) m2-Ta2N3 is 2.117 Å which is still larger than the sum of bonding radii (2.09 Å). For Ta3N5, Ta3N5-I has a lower formation enthalpy than that of Ta3N5-II, suggesting that the Ta3N5-I is thermodynamically stable at ambient condition. The calculated lattice constants of Ta3N5 are very close to the experimental values, indicating the reliability of our calculations. Obvious covalent bonding feature is found in Ta3N5-I because of the presence of the shortest Ta–N bond length (1.963 Å) among the above mentioned tantalum nitrides. We find that the bond length of Ta–N bond of tantalum nitrides decrease with the nitrogen concentration increasing (TaNx, x < 1.67). The degree of covalent bonding, which is more important for the hardness of materials, can be enhanced by increasing nitrogen concentration in tantalum nitrides. The shortest nitrogen–nitrogen distance (2.42 Å) of the above mentioned tantalum nitrides is still larger than the bond length of the single N–N bond (1.45 Å for molecule N2H4). So no nitrogen–nitrogen covalent bonds are found in these phases (TaNx, x < 1.67). However, the distinct nitrogen–nitrogen bonds chain is found in nitrogen-rich TaN3 (space group: P21/m). The shortest bond length of nitrogen–nitrogen bond (1.396 Å) is obviously smaller than the single nitrogen–nitrogen bond length of the hydrazine molecule N2H4 (1.45 Å), indicating the presence of strong covalent bonding feature in nitrogen-rich TaN3. The hardness of nitrogen-rich TaN3 could be expected to be enhanced because of the presence of strong covalent nitrogen–nitrogen bonds.
m2-Ta2N3 is 2.117 Å which is still larger than the sum of bonding radii (2.09 Å). For Ta3N5, Ta3N5-I has a lower formation enthalpy than that of Ta3N5-II, suggesting that the Ta3N5-I is thermodynamically stable at ambient condition. The calculated lattice constants of Ta3N5 are very close to the experimental values, indicating the reliability of our calculations. Obvious covalent bonding feature is found in Ta3N5-I because of the presence of the shortest Ta–N bond length (1.963 Å) among the above mentioned tantalum nitrides. We find that the bond length of Ta–N bond of tantalum nitrides decrease with the nitrogen concentration increasing (TaNx, x < 1.67). The degree of covalent bonding, which is more important for the hardness of materials, can be enhanced by increasing nitrogen concentration in tantalum nitrides. The shortest nitrogen–nitrogen distance (2.42 Å) of the above mentioned tantalum nitrides is still larger than the bond length of the single N–N bond (1.45 Å for molecule N2H4). So no nitrogen–nitrogen covalent bonds are found in these phases (TaNx, x < 1.67). However, the distinct nitrogen–nitrogen bonds chain is found in nitrogen-rich TaN3 (space group: P21/m). The shortest bond length of nitrogen–nitrogen bond (1.396 Å) is obviously smaller than the single nitrogen–nitrogen bond length of the hydrazine molecule N2H4 (1.45 Å), indicating the presence of strong covalent bonding feature in nitrogen-rich TaN3. The hardness of nitrogen-rich TaN3 could be expected to be enhanced because of the presence of strong covalent nitrogen–nitrogen bonds.
| Structure | Mag | ΔH | a | b | c | V | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| a Experiment: a (ref. 11), b (ref. 38), d (ref. 39), c (ref. 52), g (ref. 18) and i (ref. 16). Theory: e (ref. 13), f (ref. 19) and h (ref. 15). | ||||||||||
| TaN | WC | PM | −1.228 | 2.948 | 2.913a | 2.948 | 2.913a | 2.897 | 2.862a | 21.799 |
| 2.93b | 2.93b | 2.86b | ||||||||
| FM | −1.230 | 2.947 | 2.947 | 2.896 | 21.7763 | |||||
| NaCl | PM | −0.932 | 4.415 | 4.427c | 86.041 | |||||
| 4.413a | ||||||||||
| FM | −0.930 | 4.413 | 85.935 | |||||||
| CoSn | PM | −0.785 | 5.269 | 5.18a | 5.269 | 5.18a | 2.920 | 2.90a | 70.202 | |
| 5.196d | 5.196d | 2.911d | ||||||||
| 5.221a | 5.221a | 2.921a | ||||||||
| FM | −0.753 | 5.232 | 5.232 | 2.929 | 69.4287 | |||||
| Ta4N5 | I4/m | PM | −1.176 | 6.895 | 6.831e | 6.895 | 6.831e | 4.279 | 4.269e | 203.447 |
| FM | −1.135 | 6.848 | 6.848 | 4.339 | 203.505 | |||||
| Ta2N3 | P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) m2 m2 |
PM | −1.138 | 2.984 | 2.99f | 2.984 | 2.99f | 5.813 | 5.82f | 51.749 |
| FM | −1.126 | 2.984 | 2.984 | 5.810 | 51.726 | |||||
| U2S3 | PM | −1.119 | 8.227 | 8.19g | 8.180 | 8.18g | 2.996 | 2.98g | 201.585 | |
| 8.19f | 8.24f | 3.00f | ||||||||
| FM | −1.117 | 8.243 | 8.160 | 2.994 | 201.397 | |||||
| Ta3N5 | Cmcm | PM | −1.116 | 3.909 | 3.905h | 10.307 | 10.321h | 10.329 | 10.349h | 416.196 |
| 3.89i | 10.22i | 10.28i | ||||||||
| FM | −1.088 | 3.569 | 10.752 | 10.795 | 414.219 | |||||
| Pnma | PM | −0.968 | 10.978 | 10.998h | 2.960 | 2.968h | 9.594 | 9.607h | 311.704 | |
| FM | −0.953 | 10.973 | 2.968 | 9.579 | 311.902 | |||||
| TaN3 | P21/m | PM | −0.366 | 5.483 | 3.798 | 3.794 | 74.123 | |||
| FM | −0.363 | 5.482 | 3.801 | 3.789 | 74.093 | |||||
To understand the thermodynamic stability and synthesis routes of tantalum nitrides, we performed convex hull calculation for tantalum nitrides at 0 and 50 GPa. The convex hull is defined as the formation enthalpy versus composition plot. Any structure whose formation enthalpy lies on the convex hull is deemed stable and synthesizable in principle.41–45 As shown in Fig. 2(a), the convex hull is composed of five structures: Ta2N, TaN-WC, Ta5N6, Ta4N5, Ta2N3-P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) m2, and Ta3N5-I at ambient pressure. Above mentioned six structures have been observed in the experiments. For TaN, the WC-type TaN has the lowest formation enthalpy on the convex hull, indicating that the WC-type TaN is easier to be synthesized than NaCl-type and CoSn-type structures. In addition, the formation enthalpy of P
m2, and Ta3N5-I at ambient pressure. Above mentioned six structures have been observed in the experiments. For TaN, the WC-type TaN has the lowest formation enthalpy on the convex hull, indicating that the WC-type TaN is easier to be synthesized than NaCl-type and CoSn-type structures. In addition, the formation enthalpy of P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) m2-Ta2N3 lies on the convex hull, indicating the P
m2-Ta2N3 lies on the convex hull, indicating the P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) m2-Ta2N3 structure is thermodynamically stable with respect to U2S3-type Ta2N3 at ambient condition. For Ta3N5, the Ta3N5-I is thermodynamically stable with reference to Ta3N5-II at ambient condition. However, the high-pressure convex hull at 50 GPa is different from the convex hull at ambient pressure. Fig. 2(b) shows that the Fe2N-type Ta2N, WC-type TaN, U2S3-type Ta2N3, Ta3N5-II, and P21/m-TaN3 lie on the convex hull at 50 GPa. The Ta5N6 and Ta4N5 lie above the convex hull, indicating they are not thermodynamically stable at 50 GPa. The U2S3-type Ta2N3 and Ta3N5-II are energetically more stable than P
m2-Ta2N3 structure is thermodynamically stable with respect to U2S3-type Ta2N3 at ambient condition. For Ta3N5, the Ta3N5-I is thermodynamically stable with reference to Ta3N5-II at ambient condition. However, the high-pressure convex hull at 50 GPa is different from the convex hull at ambient pressure. Fig. 2(b) shows that the Fe2N-type Ta2N, WC-type TaN, U2S3-type Ta2N3, Ta3N5-II, and P21/m-TaN3 lie on the convex hull at 50 GPa. The Ta5N6 and Ta4N5 lie above the convex hull, indicating they are not thermodynamically stable at 50 GPa. The U2S3-type Ta2N3 and Ta3N5-II are energetically more stable than P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) m2-Ta2N3 and Ta3N5-I at high pressure, respectively, which is in good agreement with previous study.15,19 At 50 GPa, a novel nitrogen-rich TaN3 structure with distinct N–N bonds chain has been found. It lies on the convex hull indicating that it could be experimentally synthesized by high-pressure technique. The calculations of the phonon dispersion and elastic constants suggest that the P21/m-TaN3 is dynamically and mechanically stable at 50 GPa. The calculations of convex hull can also suggest potential synthesis routes in tantalum nitrides. It can be found that the Ta3N5-II could decompose into 3/2P
m2-Ta2N3 and Ta3N5-I at high pressure, respectively, which is in good agreement with previous study.15,19 At 50 GPa, a novel nitrogen-rich TaN3 structure with distinct N–N bonds chain has been found. It lies on the convex hull indicating that it could be experimentally synthesized by high-pressure technique. The calculations of the phonon dispersion and elastic constants suggest that the P21/m-TaN3 is dynamically and mechanically stable at 50 GPa. The calculations of convex hull can also suggest potential synthesis routes in tantalum nitrides. It can be found that the Ta3N5-II could decompose into 3/2P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) m2-Ta2N3 + 1/4N2, 3/2U2S3-type Ta2N3 + 1/4N2, or 3WC-type TaN + N2 at ambient pressure. At high pressure, the Ta3N5-I could decompose into the 3/2U2S3-type Ta2N3 + 1/4N2, 3/2P
m2-Ta2N3 + 1/4N2, 3/2U2S3-type Ta2N3 + 1/4N2, or 3WC-type TaN + N2 at ambient pressure. At high pressure, the Ta3N5-I could decompose into the 3/2U2S3-type Ta2N3 + 1/4N2, 3/2P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) m2-Ta2N3 + 1/4N2, or 3WC-type TaN + N2. The Ta4N5 (Ta5N6) could decompose into the U2S3-type Ta2N3 + 2WC-type TaN (U2S3-type Ta2N3 + 3WC-type TaN) or 4WC-type TaN + 1/2N2 (5WC-type TaN + 1/2N2). It is noteworthy that the synthesis routes of Ta3N5 → 3/2Ta2N3 + 1/4N2 and Ta3N5 → 3TaN + N2 have been confirmed by previous experiments.46,47 Two potential synthesis routes (Ta3N5 + 2N2 → 3TaN3 and Ta4N5 + 3.5N2 → 4TaN3) are also be found to synthesize nitrogen-rich TaN3. For the further study, we only consider the thermodynamically stable structures at 0 and 50 GPa, respectively.
m2-Ta2N3 + 1/4N2, or 3WC-type TaN + N2. The Ta4N5 (Ta5N6) could decompose into the U2S3-type Ta2N3 + 2WC-type TaN (U2S3-type Ta2N3 + 3WC-type TaN) or 4WC-type TaN + 1/2N2 (5WC-type TaN + 1/2N2). It is noteworthy that the synthesis routes of Ta3N5 → 3/2Ta2N3 + 1/4N2 and Ta3N5 → 3TaN + N2 have been confirmed by previous experiments.46,47 Two potential synthesis routes (Ta3N5 + 2N2 → 3TaN3 and Ta4N5 + 3.5N2 → 4TaN3) are also be found to synthesize nitrogen-rich TaN3. For the further study, we only consider the thermodynamically stable structures at 0 and 50 GPa, respectively.
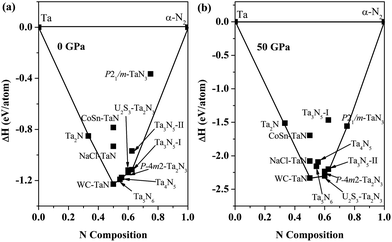 | ||
| Fig. 2 Convex hull of the Ta–N system at a pressure of (a) 0 and (b) 50 GPa. The solid line denotes the convex hull at different pressures. | ||
In order to understand the mechanical properties, the elastic constants are calculated as summarized in Table 2. It can be found that the obtained elastic constants of tantalum nitrides all satisfy the Born–Huang criterion.48 Interestingly, the P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) m2-Ta2N3 has the largest C11 and C22 (689 GPa) among tantalum nitrides which make the P
m2-Ta2N3 has the largest C11 and C22 (689 GPa) among tantalum nitrides which make the P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) m2-Ta2N3 have high incompressibility along the crystallographic a and b axis. The WC-type TaN has the largest C33 (798 GPa) value among all the tantalum nitrides which make it have high incompressibility along the crystallographic c axis. It is well known that superhard materials should have high bulk modulus and high shear modulus to resist the volume change and shape change, respectively.
m2-Ta2N3 have high incompressibility along the crystallographic a and b axis. The WC-type TaN has the largest C33 (798 GPa) value among all the tantalum nitrides which make it have high incompressibility along the crystallographic c axis. It is well known that superhard materials should have high bulk modulus and high shear modulus to resist the volume change and shape change, respectively.
| Type | P | C11 | C12 | C13 | C15 | C22 | C23 | C25 | C33 | C35 | C44 | C55 | C66 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TaN | WC | 0 | 622.5 | 213.2 | 140.2 | 614.0 | 798.0 | 233.0 | ||||||
| Ta5N6 | P63/mcm | 0 | 528 | 169.2 | 168.5 | 534.5 | 541.7 | 163.3 | ||||||
| Ta4N5 | I4/m | 0 | 536.3 | 168.6 | 122.5 | 536.3 | 751.5 | 138.0 | 167.5 | |||||
| Ta2N3 | P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) m2 m2 |
0 | 689.0 | 146.8 | 178.5 | 689.0 | 607.0 | 144.3 | 1683.3 | |||||
| Ta3N5 | Cmcm | 0 | 393.5 | 206.2 | 166.3 | 391.0 | 127.3 | 385.0 | 114.0 | 120.3 | 58.3 | |||
| TaN | WC | 50 | 910.8 | 366.2 | 295.7 | 898.7 | 1126.5 | 344.3 | ||||||
| Ta2N3 | U2S3 | 50 | 728.3 | 415.5 | 381.2 | 962.2 | 899.7 | 249.3 | 186.0 | |||||
| Ta3N5 | Pnma | 50 | 901.5 | 321.8 | 373.8 | 1025.2 | 313.3 | 935.7 | 230.8 | 271.1 | 238.0 | |||
| TaN3 | P21/m | 50 | 620.0 | 177.2 | 197.5 | 0 | 401.8 | 193.2 | 0 | 612.0 | 0 | 123.0 | 145.7 | 146.2 |
As listed in Table 3, tantalum nitrides have high bulk moduli, which indicate that they are difficult to be compressed. At ambient condition, the bulk moduli follow the descending order WC-type TaN > P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) m2-Ta2N3 > Ta4N5 > Ta5N6 > Ta3N5-I. The WC-type TaN has the largest bulk modulus (337.9 GPa) among all the tantalum nitrides. Generally, bulk modulus might have a direct correlation with valence electron densities.49,50 So we investigate the average valence electron density (VED) for tantalum nitrides. It is found that the VED values of WC-type TaN (0.73 electrons per Å3), P
m2-Ta2N3 > Ta4N5 > Ta5N6 > Ta3N5-I. The WC-type TaN has the largest bulk modulus (337.9 GPa) among all the tantalum nitrides. Generally, bulk modulus might have a direct correlation with valence electron densities.49,50 So we investigate the average valence electron density (VED) for tantalum nitrides. It is found that the VED values of WC-type TaN (0.73 electrons per Å3), P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) m2-Ta2N3 (0.71 electrons per Å3), Ta5N6 (0.688 electrons per Å3), Ta4N5 (0.68 electrons per Å3), and Ta3N5-I (0.56 electrons per Å3) are following the similar tendency as the variation of bulk moduli. In contrast, at 50 GPa, the bulk moduli of tantalum nitrides follow the descending order WC-type TaN > U2S3-type Ta2N3 > P21/m-TaN3 > Ta3N5. The WC-type TaN structure still has the largest bulk modulus (336.7 GPa) among the stable high-pressure structures. The Ta3N5-II has the smallest bulk modulus (241.0 GPa). The VED values of tantalum nitrides follow the descending order Ta3N5-II (0.832 electrons per Å3) > U2S3-type Ta2N3 (0.823 electrons per Å3) > WC-type TaN (0.818 electrons per Å3) > TaN3 (0.798 electrons per Å3) at high pressure. It is noteworthy that the variation of the valence electron density does not follow the same tendency as the variation of bulk moduli at high pressure. It is different from the result at ambient pressure. The WC-type TaN also has the largest shear modulus (239.7 GPa and 244.5 GPa at 0 and 50 GPa, respectively) among the tantalum nitrides. The P
m2-Ta2N3 (0.71 electrons per Å3), Ta5N6 (0.688 electrons per Å3), Ta4N5 (0.68 electrons per Å3), and Ta3N5-I (0.56 electrons per Å3) are following the similar tendency as the variation of bulk moduli. In contrast, at 50 GPa, the bulk moduli of tantalum nitrides follow the descending order WC-type TaN > U2S3-type Ta2N3 > P21/m-TaN3 > Ta3N5. The WC-type TaN structure still has the largest bulk modulus (336.7 GPa) among the stable high-pressure structures. The Ta3N5-II has the smallest bulk modulus (241.0 GPa). The VED values of tantalum nitrides follow the descending order Ta3N5-II (0.832 electrons per Å3) > U2S3-type Ta2N3 (0.823 electrons per Å3) > WC-type TaN (0.818 electrons per Å3) > TaN3 (0.798 electrons per Å3) at high pressure. It is noteworthy that the variation of the valence electron density does not follow the same tendency as the variation of bulk moduli at high pressure. It is different from the result at ambient pressure. The WC-type TaN also has the largest shear modulus (239.7 GPa and 244.5 GPa at 0 and 50 GPa, respectively) among the tantalum nitrides. The P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) m2-Ta2N3 has the second larger shear modulus (190.2 GPa) at ambient condition. The Ta3N5-I has the highest nitrogen concentration among the experimentally observed tantalum nitrides. However, the shear modulus of Ta3N5-I is the smallest one among all the relevant structures. The Poisson's ratio and B/G ratio are also two important parameters to describe the mechanical properties except the bulk modulus and shear modulus. The Poisson's ratio is indicator of the degree of directionality of the covalent bonding. Lower Poisson's ratio (about 0.2) indicates that the directionality of the covalent bonding is good in materials.51 The B/G value is associated with the ductility (brittleness) of materials, and the critical value is about 1.75. The Poisson's ratio v was obtained by the following formula:
m2-Ta2N3 has the second larger shear modulus (190.2 GPa) at ambient condition. The Ta3N5-I has the highest nitrogen concentration among the experimentally observed tantalum nitrides. However, the shear modulus of Ta3N5-I is the smallest one among all the relevant structures. The Poisson's ratio and B/G ratio are also two important parameters to describe the mechanical properties except the bulk modulus and shear modulus. The Poisson's ratio is indicator of the degree of directionality of the covalent bonding. Lower Poisson's ratio (about 0.2) indicates that the directionality of the covalent bonding is good in materials.51 The B/G value is associated with the ductility (brittleness) of materials, and the critical value is about 1.75. The Poisson's ratio v was obtained by the following formula:
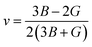 | (1) |
| Type | P | B | G | B/G | Y | v | Hv | |
|---|---|---|---|---|---|---|---|---|
| TaN | WC | 0 | 337.9 | 239.7 | 1.41 | 581.7 | 0.21 | 30.0 |
| Ta5N6 | P63/mcm | 0 | 290.4 | 175.2 | 1.65 | 437.5 | 0.25 | 19.7 |
| Ta4N5 | I4/m | 0 | 294.6 | 182.7 | 1.61 | 454.3 | 0.24 | 21.1 |
| Ta2N3 | P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) m2 m2 |
0 | 332.5 | 190.2 | 1.75 | 479.2 | 0.26 | 19.4 |
| Ta3N5 | Cmcm | 0 | 241.0 | 103.2 | 2.32 | 270.9 | 0.31 | 8.2 |
| TaN | WC | 50 | 336.7 | 244.5 | 1.37 | 590.4 | 0.21 | 31.3 |
| Ta2N3 | U2S3 | 50 | 332.5 | 190.1 | 1.75 | 479.1 | 0.26 | 19.4 |
| Ta3N5 | Pnma | 50 | 241.0 | 103.2 | 2.32 | 270.8 | 0.31 | 8.2 |
| TaN3 | P21/m | 50 | 307.7 | 154.0 | 2 | 396.0 | 0.29 | 14 |
Table 3 reflects that the ascending order of Poisson's ratio is WC-type TaN < Ta4N5 (Ta5N6) < P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) m2-Ta2N3 < Ta3N5-I at ambient condition. It is found that the degree of directionality of the covalent bonding can reduce with the nitrogen content increasing in tantalum nitrides. The WC-type TaN, Ta4N5, Ta5N6 and P
m2-Ta2N3 < Ta3N5-I at ambient condition. It is found that the degree of directionality of the covalent bonding can reduce with the nitrogen content increasing in tantalum nitrides. The WC-type TaN, Ta4N5, Ta5N6 and P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) m2-Ta2N3 have smaller Poisson's ratios (∼0.25), implying higher degree of directionality of covalent bonding in those structures. The variation of Poisson's ratio at 50 GPa is similar to that of Poisson's ratio at ambient pressure. However, when the nitrogen content reaches to x = 3 in TaNx, the Poisson's ratio of TaN3 decreases larger than that of the preceding composition Ta3N5-II. The relative directionality of covalent bonds has an important effect on the hardness of materials. So we can estimate that the hardness of tantalum nitrides decreases with the nitrogen contents increasing. The following hardness calculations confirm this point. The Vickers hardness Hv of tantalum nitrides is estimated by recently proposed empirical model Hv = 2.0(k2G)0.585–3.0 (Hv in GPa),14 which has better results for the anisotropic structures. The variation of hardness has the opposite tendency to that of Poisson's ratio in tantalum nitrides as listed in the Table 3. The WC-type TaN has the highest hardness (30 GPa). The hardness of Ta3N5-I is only 8.2 GPa at 0 GPa. At high pressure, when the nitrogen composition reaches to x = 3 in TaNx, the Poisson's ratio decreases while the hardness of TaN3 (14 GPa) abnormally increases with respect to that of the preceding composition Ta3N5-II (8.2 GPa) because of the presence of the strong covalent nitrogen–nitrogen bonds chain along the crystallographic b axis in TaN3. The higher nitrogen concentration is the key for the formation and directionality of strong covalent nitrogen–nitrogen bonds, which make the tantalum nitrides have good mechanical properties. With the nitrogen content increasing, the variation of B/G has the same tendency as the variation of Poisson's ratio. The B/G value of Ta2N3 lies on the critical value (1.75). The WC-type TaN, Ta5N6 and Ta4N5 have small B/G values (1.41, 1.65 and 1.61, respectively), which indicates they are brittle. The B/G value of Ta3N5-I (2.32) is much larger than the critical value, reflecting it is a good ductile material. It is found that the ductility of tantalum nitrides can also be effectively improved by increasing the nitrogen content.
m2-Ta2N3 have smaller Poisson's ratios (∼0.25), implying higher degree of directionality of covalent bonding in those structures. The variation of Poisson's ratio at 50 GPa is similar to that of Poisson's ratio at ambient pressure. However, when the nitrogen content reaches to x = 3 in TaNx, the Poisson's ratio of TaN3 decreases larger than that of the preceding composition Ta3N5-II. The relative directionality of covalent bonds has an important effect on the hardness of materials. So we can estimate that the hardness of tantalum nitrides decreases with the nitrogen contents increasing. The following hardness calculations confirm this point. The Vickers hardness Hv of tantalum nitrides is estimated by recently proposed empirical model Hv = 2.0(k2G)0.585–3.0 (Hv in GPa),14 which has better results for the anisotropic structures. The variation of hardness has the opposite tendency to that of Poisson's ratio in tantalum nitrides as listed in the Table 3. The WC-type TaN has the highest hardness (30 GPa). The hardness of Ta3N5-I is only 8.2 GPa at 0 GPa. At high pressure, when the nitrogen composition reaches to x = 3 in TaNx, the Poisson's ratio decreases while the hardness of TaN3 (14 GPa) abnormally increases with respect to that of the preceding composition Ta3N5-II (8.2 GPa) because of the presence of the strong covalent nitrogen–nitrogen bonds chain along the crystallographic b axis in TaN3. The higher nitrogen concentration is the key for the formation and directionality of strong covalent nitrogen–nitrogen bonds, which make the tantalum nitrides have good mechanical properties. With the nitrogen content increasing, the variation of B/G has the same tendency as the variation of Poisson's ratio. The B/G value of Ta2N3 lies on the critical value (1.75). The WC-type TaN, Ta5N6 and Ta4N5 have small B/G values (1.41, 1.65 and 1.61, respectively), which indicates they are brittle. The B/G value of Ta3N5-I (2.32) is much larger than the critical value, reflecting it is a good ductile material. It is found that the ductility of tantalum nitrides can also be effectively improved by increasing the nitrogen content.
The electronic structure is crucial to understand the origin of physical properties of these nitrides. The total and partial density of states (PDOS) of the structures lied on the convex hull at different pressures are shown in Fig. 3. Fig. 3(a) shows that WC-type TaN, Ta5N6, Ta4N5, and P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) m2-Ta2N3 are metallic because of their finite electron DOS at the Fermi level at 0 GPa. The Ta3N5-I is a semiconductor with band gap of 1.2 eV. The whole DOS curves are composed of three parts in the Ta–N system. For WC-type TaN, three parts are in range of −8.8–−3.3 eV, −3.3–0 eV and 0–7.5 eV. It can be found that the second part is close to the Fermi level and is moving to the high energy range with the nitrogen content increasing. The intensity of the second part is decreasing with the nitrogen content increasing as shown in the zone surrounded by the green dot line. The second part disappears while the Ta
m2-Ta2N3 are metallic because of their finite electron DOS at the Fermi level at 0 GPa. The Ta3N5-I is a semiconductor with band gap of 1.2 eV. The whole DOS curves are composed of three parts in the Ta–N system. For WC-type TaN, three parts are in range of −8.8–−3.3 eV, −3.3–0 eV and 0–7.5 eV. It can be found that the second part is close to the Fermi level and is moving to the high energy range with the nitrogen content increasing. The intensity of the second part is decreasing with the nitrogen content increasing as shown in the zone surrounded by the green dot line. The second part disappears while the Ta![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N ratio reach to 1
N ratio reach to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.67. The tantalum nitrides transform from metal to semiconductor with the nitrogen content increasing. At 50 GPa, the result shows that the variation of the second part follow the same trend as that at 0 GPa as shown in Fig. 3(b). It is decreasing with the nitrogen content increasing. The pressure makes the Ta3N5-II has metallic properties due to finite electron DOS at the Fermi level. However, when the nitrogen composition reaches to x = 3 in TaNx, the second part of TaN3 abnormally increases with reference to that of the preceding composition Ta3N5-II because of the unpaired d electron of tantalum atoms in TaN3. Generally, the DFT underestimates band gap. However, this does not change the trend of variation. So the metallic properties of tantalum nitrides can be effectively adjusted by controlling the nitrogen content and pressure in tantalum nitrides.
1.67. The tantalum nitrides transform from metal to semiconductor with the nitrogen content increasing. At 50 GPa, the result shows that the variation of the second part follow the same trend as that at 0 GPa as shown in Fig. 3(b). It is decreasing with the nitrogen content increasing. The pressure makes the Ta3N5-II has metallic properties due to finite electron DOS at the Fermi level. However, when the nitrogen composition reaches to x = 3 in TaNx, the second part of TaN3 abnormally increases with reference to that of the preceding composition Ta3N5-II because of the unpaired d electron of tantalum atoms in TaN3. Generally, the DFT underestimates band gap. However, this does not change the trend of variation. So the metallic properties of tantalum nitrides can be effectively adjusted by controlling the nitrogen content and pressure in tantalum nitrides.
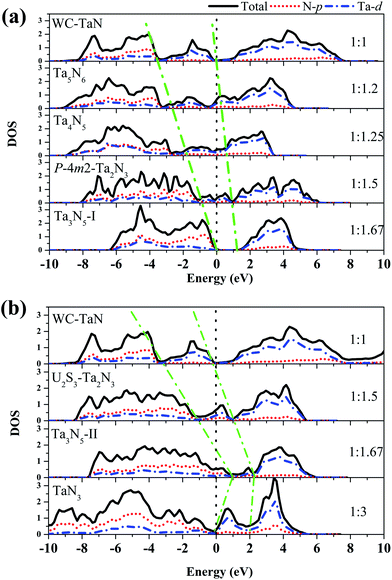 | ||
| Fig. 3 The density of states of the TaNx (x ≥ 1) at 0 (a) and 50 GPa (b). All the structures are simplified to one Ta atom and x N atoms. The black dot line denotes the fermi level. | ||
4 Conclusions
The phase stability, mechanical properties and metallic properties of tantalum nitrides (TaNx, x ≥ 1) are investigated by first principles calculations. We uncover a synthesizable new composition TaN3 with strong covalent N–N bonds chains along the crystallographic b axis. The established enthalpy versus composition phase diagram of tantalum nitrides is of fundamental interest and important for future experimental synthesis. With the nitrogen concentration increasing, the feature of covalent bonding of tantalum nitrides can be enhanced while the degree of directionality of the covalent bonding and hardness reduce. These make the ductility of the tantalum nitrides improved with the nitrogen composition increasing. However, the hardness of the nitrogen-rich TaN3 increases obviously with respect to that of the preceding composition Ta3N5-I because of the presence of remarkable nitrogen–nitrogen bonds in TaN3. Moreover, the intensity of metallic properties of tantalum nitrides can be effectively adjusted by controlling nitrogen composition and pressure. We hope that these calculations will stimulate extensive experimental work on these technologically important tantalum nitrides.Acknowledgements
This work was supported by the National Basic Research Program of China (No. 2011CB808200), Program for Changjiang Scholars and Innovative Research Team in University (no. IRT1132), National Natural Science Foundation of China (no. 51032001, 11074090, 10979001, 51025206), and National Found for Fostering Talents of basic Science (no. J1103202). Parts of calculations were performed in the High Performance Computing Center (HPCC) of Jilin University.Notes and references
- F. Occelli, P. Loubeyre and R. LeToullec, Nat. Mater., 2003, 2, 151–154 CrossRef CAS PubMed.
- J.-C. Zheng, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 72, 052105 CrossRef.
- J. He, L. Guo, X. Guo, R. Liu, Y. Tian, H. Wang and C. Gao, Appl. Phys. Lett., 2006, 88, 101906–101903 CrossRef PubMed.
- V. L. Solozhenko, D. Andrault, G. Fiquet, M. Mezouar and D. C. Rubie, Appl. Phys. Lett., 2001, 78, 1385–1387 CrossRef CAS PubMed.
- V. L. Solozhenko, O. O. Kurakevych, D. Andrault, Y. Le Godec and M. Mezouar, Phys. Rev. Lett., 2009, 102, 015506 CrossRef.
- E. Gregoryanz, C. Sanloup, M. Somayazulu, J. Badro, G. Fiquet, H. K. Mao and R. J. Hemley, Nat. Mater., 2004, 3, 294–297 CrossRef CAS PubMed.
- J. C. Crowhurst, A. F. Goncharov, B. Sadigh, C. L. Evans, P. G. Morrall, J. L. Ferreira and A. J. Nelson, Science, 2006, 311, 1275–1278 CrossRef CAS PubMed.
- A. F. Young, C. Sanloup, E. Gregoryanz, S. Scandolo, R. J. Hemley and H.-k. Mao, Phys. Rev. Lett., 2006, 96, 155501 CrossRef.
- G. S. Chen, P. Y. Lee and S. T. Chen, Thin Solid Films, 1999, 353, 264–273 CrossRef CAS.
- T. Chihi, J. C. Parlebas and M. Guemmaz, Phys. Status Solidi B, 2011, 248, 2787–2792 CrossRef CAS.
- C. L. Cao, Z. F. Hou and G. Yuan, Phys. Status Solidi B, 2008, 245, 1580–1585 CrossRef CAS.
- T. Mashimo, S. Tashiro, M. Nishida, K. Miyahara and E. Eto, Phys B Condens Matter, 1997, 239, 13–15 CrossRef CAS.
- C. Stampfl and A. Freeman, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 67, 064108 CrossRef.
- X.-Q. Chen, H. Niu, D. Li and Y. Li, Intermetallics, 2011, 19, 1275–1281 CrossRef CAS PubMed.
- P. Kroll, T. Schroter and M. Peters, Angew. Chem., Int. Ed., 2005, 44, 4249–4254 CrossRef CAS PubMed.
- S. J. Henderson and A. L. Hector, J. Solid State Chem., 2006, 179, 3518–3524 CrossRef CAS PubMed.
- E. Horvath-Bordon, R. Riedel, A. Zerr, P. F. McMillan, G. Auffermann, Y. Prots, W. Bronger, R. Kniep and P. Kroll, Chem. Soc. Rev., 2006, 35, 987–1014 RSC.
- A. Zerr, G. Miehe, J. Li, D. A. Dzivenko, V. K. Bulatov, H. Höfer, N. Bolfan-Casanova, M. Fialin, G. Brey, T. Watanabe and M. Yoshimura, Adv. Funct. Mater., 2009, 19, 2282–2288 CrossRef CAS.
- C. Jiang, Z. Lin and Y. Zhao, Phys. Rev. Lett., 2009, 103, 185501 CrossRef.
- L. G. Boiko and S. V. Popova, JETP Lett., 1970, 12, 70 Search PubMed.
- R. Kieffer and P. Ettmayer, Chem. Ing. Tech., 1974, 46, 843–852 CrossRef CAS.
- Z. Fang, H. C. Aspinall, R. Odedra and R. J. Potter, J. Cryst. Growth, 2011, 331, 33–39 CrossRef CAS PubMed.
- A. R. Oganov, C. W. Glass and S. Ono, Earth Planet. Sci. Lett., 2006, 241, 95–103 CrossRef CAS PubMed.
- A. R. Oganov and C. W. Glass, J. Chem. Phys., 2006, 124, 244704 CrossRef PubMed.
- C. W. Glass, A. R. Oganov and N. Hansen, Comput. Phys. Commun., 2006, 175, 713–720 CrossRef CAS PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS.
- G. Kresse and D. Joubert, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758–1775 CrossRef CAS.
- G. Kresse and J. Furthmüller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS.
- P. E. Blöchl, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953–17979 CrossRef.
- H. J. Monkhorst and J. D. Pack, Phys. Rev. B: Condens. Matter Mater. Phys., 1976, 13, 5188–5192 CrossRef.
- K. Parlinski, http://wolf.ifj.edu.pl/phonon.
- R. Hill, Proc. Phys. Soc., London, Sect. A, 1952, 65, 349–354 CrossRef.
- W. Voigt, Lehrburch der Kristallphysik, Teubner, Leipzig, 1928 Search PubMed.
- A. Reuss, Z. Angew. Math. Mech., 1929, 9, 49–58 CrossRef CAS.
- D. Li, K. Bao, F. Tian, Z. Zeng, Z. He, B. Liu and T. Cui, Phys. Chem. Chem. Phys., 2012, 14, 4347–4350 RSC.
- F. B. Tian, J. H. Wang, Z. He, Y. M. Ma, L. C. Wang, T. Cui, C. B. Chen, B. B. Liu and G. T. Zou, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 78, 235431 CrossRef.
- D. Li, K. Bao, F. Tian, X. Jin, D. Duan, Z. He, B. Liu and T. Cui, RSC Adv., 2014, 4, 203–207 RSC.
- N. Terao, Jpn. J. Appl. Phys., 1971, 10, 259 Search PubMed.
- A. N. Christensen and B. Lebech, Acta Crystallogr., Sect. B: Struct. Sci., 1978, 34, 261–263 CrossRef.
- E. Kurmaev, A. Moewes, Z. Pchelkina, I. Nekrasov, A. Rempel and D. Ederer, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 64, 073108 CrossRef.
- X. Zhang, G. Trimarchi and A. Zunger, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 79, 092102 CrossRef.
- G. Ghosh, A. van de Walle and M. Asta, Acta Mater., 2008, 56, 3202–3221 CrossRef CAS PubMed.
- M. Zhang, H. Wang, H. Wang, T. Cui and Y. Ma, J. Phys. Chem. C, 2010, 114, 6722–6725 CAS.
- B. Wang, X. Li, Y. X. Wang and Y. F. Tu, J. Phys. Chem. C, 2011, 115, 21429–21435 CAS.
- E. Zhao, J. Meng, Y. Ma and Z. Wu, Phys. Chem. Chem. Phys., 2010, 12, 13158–13165 RSC.
- A. Zerr, G. Miehe, J. Li, D. A. Dzivenko, V. K. Bulatov, H. Höfer, N. Bolfan-Casanova, M. Fialin, G. Brey, T. Watanabe and M. Yoshimura, Adv. Funct. Mater., 2009, 19, 2282–2288 CrossRef CAS.
- B. Fu and L. Gao, J. Am. Ceram. Soc., 2005, 88, 3519–3521 CrossRef CAS.
- Z.-J. Wu, E.-J. Zhao, H.-P. Xiang, X.-F. Hao, X.-J. Liu and J. Meng, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 054115 CrossRef.
- J. J. Gilman, R. W. Cumberland and R. B. Kaner, Int. J. Refract. Met. Hard Mater., 2006, 24, 1–5 CrossRef CAS PubMed.
- C. Li and P. Wu, Chem. Mater., 2001, 13, 4642–4648 CrossRef CAS.
- J. Haines, J. Léger and G. Bocquillon, Annu. Rev. Mater. Res., 2001, 31, 1–23 CrossRef CAS.
- E. Z. Kurmaev, A. Moewes, Z. V. Pchelkina, I. A. Nekrasov, A. A. Rempel and D. L. Ederer, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 64, 073108 CrossRef.
| This journal is © The Royal Society of Chemistry 2014 |