Colloidal synthesis of ultrathin γ-Fe2O3 nanoplates†
Abstract
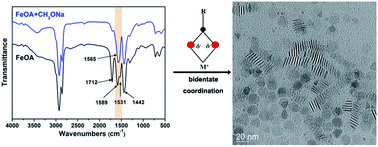
A facile method of synthesizing γ-Fe2O3 ultrathin nanoplates has been developed. These nanoplates are single crystalline and superparamagnetic at room temperature, with a thickness of only 1.4 nm. FTIR analysis has shown that the coordination mode between Fe and carboxyl group is dominated by bidentate configuration in the as prepared iron oleate complex, which is the key for producing the nanoplate morphology. By changing the reaction temperatures, the lateral size and thickness of nanoplates can be varied.

 Please wait while we load your content...
Please wait while we load your content...