Transition of chemically modified diphenylalanine peptide assemblies revealed by atomic force microscopy†
Abstract
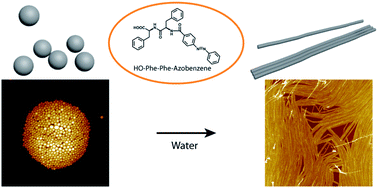
Self-assembled structures from aromatic dipeptides have attracted a lot of attention. It is highly desirable to produce dipeptide assemblies which undergo structural transitions in response to external stimuli. In this paper, solid nanospheres were successful produced from the self-assembly of chemically modified diphenylalanine in hexafluoroisopropanol (HFIP), a highly polar solvent. Interestingly, after treatment with water, the nanospheres were transformed into nanofibers. The intermediate transition state of nanospheres embedded along the nanofibers was captured by atomic force microscopy (AFM) imaging. In addition, AFM-based nanomechanical measurement revealed the increased stiffness after the transition, suggesting enhanced molecular packing due to favoured intermolecular interactions in water. This study presents a new method to fabricate novel dipeptide structures and provides new information for understanding the mechanism of dipeptide self-assembly driving by intermolecular interactions.

 Please wait while we load your content...
Please wait while we load your content...