Nanofibers to nanocuboids of polyaniline by lead nitrate: hierarchical self-assembly with lead ions†
Abstract
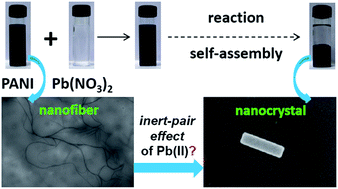
By mixing the solutions of polyaniline base and lead nitrate together an oxidized form of polyaniline was gradually precipitated-out together with toxic lead ions under ambient conditions. A remarkable morphological transformation of polyaniline nanofibers to nanocuboids with lead ions suggested a spontaneous and hierarchical self-assembly. We have attributed such in situ formation of supramolecular nanomaterial to the specific influence of relativistic inert-pair effects of the lead ions. Our exploration of one of the relativistic quantum effects in generating supramolecular nanomaterials are thought-provoking and pave the way toward designing a new type of self-assembled materials through which toxic heavy metal ions are automatically removed from aqueous solution.

 Please wait while we load your content...
Please wait while we load your content...