Free standing silica thin films with highly ordered perpendicular nanopores†
Abstract
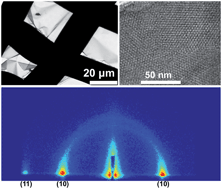
The synthesis of mesoporous silica thin films on nontraditional conductive substrates using an electro-assisted self-assembly (EASA) technique is described. This work extends prior demonstrations of EASA by exploring the effects of precursor sol pH, temperature, and substrate roughness and describes a new approach to synthesizing a mesoporous silica film that is detachable from the substrate. The latter uses a conductive polymer as a planarization layer for successful EASA on indium-tin oxide coated glass (ITO) and as a sacrificial layer that can be subsequently dispersed in water to release the silica film. This is a particularly important development because it opens up possibilities for synthesizing perpendicularly aligned nanoporous silica on a broad range of surfaces and non-conductive substrates and producing free standing nanostructured thin films. The silica films that are produced have well ordered hexagonally packed mesopores that are vertical to the substrate surface. The thicknesses of these mesoporous silica films were examined by scanning electron microscopy (SEM) and determined to be controllably variable between ∼100 and 200 nm. Transmission electron microscopy (TEM) showed organized porous structural features that were approximately 3 nm in diameter. Grazing-incidence small angle X-ray scattering (GISAXS) analysis yielded an ∼4.2 nm pore-to-pore distance and confirmed that a p6mm orientation persisted throughout the 6 cm2 mesoporous silica film samples.

 Please wait while we load your content...
Please wait while we load your content...