Temperature dependent 2D self-assembled motif transition of copper–phthalocyanine derivates at air/HOPG interface: an STM study†
Abstract
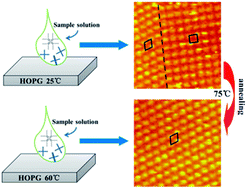
It is known that copper(II) 2,3,9,10,16,17,23,24-octakis(octyloxy)-29H,31H-phthalocyanine (CuPcOC8) molecules can self-organize on highly oriented pyrolytic graphite and form both quartic symmetry structures with relatively low molecular packing density and hexagonal symmetry structures with high packing density. However, the mechanism of the formation of the two types of molecular adlayers, and the tunability of this metal phthalocyanine film in ambient atmosphere has not been discussed. In this work, by increasing the temperature during the self-assembly process, or applying an annealing treatment to the self-assembled film, the transition of the self-assembled structure from quartic to hexagonal symmetry was observed. With images obtained with scanning tunnelling microscopy under different conditions and statistical analysis of the ratios of the quartic to hexagonal domain areas, this work revealed the existence of an energy barrier between the quartic and the hexagonal structures. The hexagonal phase corresponds to a thermodynamically stable state while the quartic phase is kinetically favorable but thermodynamically metastable. It was also found that with high enough temperature and given enough time, uniform ordered assembly motifs of CuPcOC8 molecules on a large scale can be obtained.

 Please wait while we load your content...
Please wait while we load your content...