A visible light excitable pyrene–naphthalene conjugate for ON fluorescence sensing of histidine in living cells†
Sudipta Dasa,
Animesh Sahanaa,
Sisir Lohara,
Bidisha Sarkarb,
Subhra Kanti Mukhopadhyab,
Arnab Banerjee*a and
Debasis Das*a
aDepartment of Chemistry, The University of Burdwan, Burdwan, 713104, West Bengal, India. E-mail: arnab.ism@gmail.com; ddas100in@yahoo.com; Fax: +91 342 2530452; Tel: +91 342 2533913
bDepartment of Microbiology, The University of Burdwan, Burdwan 713104, India
First published on 10th January 2014
Abstract
Pyrene appended naphthalene derivative, NEDAP can selectively detect histidine via inter-molecular H-bond assisted molecular ring formation that imparts rigidity to the molecular assembly to inhibit the TICT (Twisted Intra-molecular Charge Transfer) process leading to fluorescence enhancement. 1H NMR titration, mass spectra and DFT (Density Functional Theory) studies nicely demonstrate this sensing process. Visible light excitation favours the living cell imaging as low as 0.01 μM histidine.
Introduction
Histidine is an essential amino acid for human growth1 function as a neurotransmitter or neuromodulator in mammalian central nervous system.2,3 The imidazole unit of histidine is the active site in metallo-proteins and enzymes.4 Histidine deficiency causes epilepsy, Parkinson's disease and normal erythropoiesis.5 On the other hand, excess accumulation of histidine causes histidinemia.6 Thus, trace level determination of histidine in the human body has immense research interest. Several methods viz. capillary electrophoresis7,8 liquid chromatography9,10 voltammetry11 and spectrophotometry12–16 are available for histidine determination. However, they require either sophisticated equipment or long analysis time or lack accuracy at sub-ppm levels for in vivo application. Fluorescence method overcomes these difficulties. Most of the available fluorescent probes for histidine17–22 require UV-light excitation, and hence inappropriate for in vivo studies. Visible light excitation can minimize sample damage and native auto-fluorescence events associated with UV excitation. Our attempt towards development of low cost, visible light excitable fluorescence probes23–26 for selective monitoring of in vivo histidine have resulted the probe NEDAP, in which pyrene is chosen as visible light excitable fluorophore. Intermolecular H-bonding assisted on-fluorescence sensing has become an important technique in recent years.24b–e Well known basic N-center of imidazole unit of histidine has the required proton affinity27 to form intermolecular H-bond with –NH part of NEDAP. On the other hand, the acidic proton of histidine forms H-bond with imine N-center of NEDAP; leading to the formation of a molecular ring.Results and discussion
Synthesis and spectral characteristics
NEDAP is synthesized (Scheme 1) by a facile one step condensation between N1-(naphthalen-1-yl)ethane-1,2-diamine hydrochloride with pyrene-1-carbaldehyde in methanol (details are in the ESI†).A DMSO–water (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
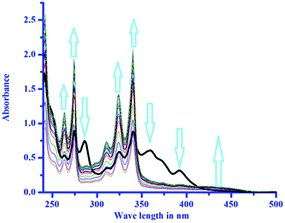
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v, pH 7.4) solution of NEDAP has absorbances at 393 nm, 360 nm, 340 nm, and 325 nm (Fig. 1).
3, v/v, pH 7.4) solution of NEDAP has absorbances at 393 nm, 360 nm, 340 nm, and 325 nm (Fig. 1).
Aqueous DMSO solution is chosen to compare the results with TD-DFT studies.26 Gradual addition of histidine to NEDAP produces a new band at 435 nm with simultaneous disappearance of two other peaks viz. 393 nm and 360 nm. The absorbance at 340 nm and 325 nm enhances with increasing histidine concentration.
NEDAP in HEPES buffered solution exhibits very weak fluorescence at 458 nm (λEx = 410 nm, Φ = 0.05). Gradual addition of histidine (up to 10 equivalent) causes a maximum of ∼15-fold fluorescence enhancement (Φ = 0.26) (Fig. 2). Plot of emission intensity as a function of the histidine concentration results a gradual increase of emission intensity up to almost 1 μM histidine after which no significant change in the emission intensity is observed (Fig. S1†).
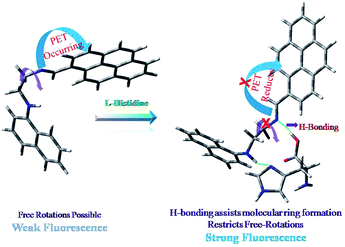
The linear part is useful to determine unknown histidine concentration (Fig. S1 inset, ESI†). Most of the bio-relevant cations and anions either do not affect or quench the emission intensity to some extent (Fig. 3). Except lysine and arginine (∼2 fold fluorescence enhancement), other naturally occurring amino acids do not show any significant change in the emission intensity of NEDAP in a binary mixture. Very weak fluorescence of NEDAP is attributed to the PET (Photo-induce Electron Transfer) process23e,28 from imine N-center and associated TICT24d,29 due to free rotation around imine (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) bond.
N–) bond.
In presence of histidine, NEDAP forms a molecular ring through inter-molecular H-bonding between the basic N-atom of imidazole ring of histidine with –NH in NEDAP and the acid proton of histidine with the imine N-center (Fig. 4). Formation of molecular ring provides rigidity to the system, which inhibits the TICT process by restricting the free rotation around-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– bond. Due to involvement of imine N-center in H-bond formation, the PET process is also inhibited. Combination of these two processes is responsible for fluorescence enhancement. Except basic amino acids (viz. histidine, lysine and arginine), no other amino acid is capable to form such type of molecular ring with NEDAP due to the absence of basic N-center, and hence no fluorescence enhancement is observed. Again, amongst basic amino acids, the pKa value of the basic N-center is lowest for histidine (histidine, 6.04; lysine, 10.79 and arginine, 12.48). Hence, the ability to form intermolecular H-bonding with –NH center of NEDAP is maximum for histidine. Combining these two facts, one can easily rationalize the fluorescence enhancement (∼15 fold) of NEDAP in presence of histidine. Fig. 5 shows the effect of other naturally occurring amino acids on the emission intensity of [NEDAP–His] adduct. No interference is observed. Moreover, addition of imidazole in a solution of NEDAP–amino acid mixture (excluding basic amino acids) causes no significant changes in the emission intensity. This fact strongly supports our proposed mechanism of H-bond assisted molecular ring forming.
N– bond. Due to involvement of imine N-center in H-bond formation, the PET process is also inhibited. Combination of these two processes is responsible for fluorescence enhancement. Except basic amino acids (viz. histidine, lysine and arginine), no other amino acid is capable to form such type of molecular ring with NEDAP due to the absence of basic N-center, and hence no fluorescence enhancement is observed. Again, amongst basic amino acids, the pKa value of the basic N-center is lowest for histidine (histidine, 6.04; lysine, 10.79 and arginine, 12.48). Hence, the ability to form intermolecular H-bonding with –NH center of NEDAP is maximum for histidine. Combining these two facts, one can easily rationalize the fluorescence enhancement (∼15 fold) of NEDAP in presence of histidine. Fig. 5 shows the effect of other naturally occurring amino acids on the emission intensity of [NEDAP–His] adduct. No interference is observed. Moreover, addition of imidazole in a solution of NEDAP–amino acid mixture (excluding basic amino acids) causes no significant changes in the emission intensity. This fact strongly supports our proposed mechanism of H-bond assisted molecular ring forming.
 | ||
Fig. 5 Effect of other amino acids (5 μM) on the emission intensity of [NEDAP–His] molecular assembly (1 μM) in a ternary mixture in HEPES buffer (0.1 M) (DMSO–water, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v, pH 7.4). 3, v/v, pH 7.4). | ||
Job's plot reveals the stoichiometry of the [NEDAP–His] adduct as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (NEDAP
1 (NEDAP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) His, mole ratio) (Fig. S2†) while its mass spectrum (Fig. S3†) have also supported the composition. Binding interaction of NEDAP with Histidine has been estimated using the modified Benesi–Hildebrand equation30 as presented below:
His, mole ratio) (Fig. S2†) while its mass spectrum (Fig. S3†) have also supported the composition. Binding interaction of NEDAP with Histidine has been estimated using the modified Benesi–Hildebrand equation30 as presented below:
| (ΔFmax/ΔF) = 1 + (1/K[C]n) | (i) |
 | (ii) |
here, F is fluorescence intensity at any [His], F0 is fluorescence intensity of free NEDAP, CD and CP are the concentration. of NEDAP and histidine respectively. The plot of [(F – F0)/CD] vs. CP have yielded a sigmoidal curve (Fig. S5†). Association constant, K obtained from the plot is 1.214 × 106 M−1, which is in close agreement with the above value obtained from modified Benesi–Hildebrand equation.
The same plot has also been used to estimate the lowest detection limit of histidine as 0.01 μM, a value that falls within the normal histidine level in human beings (details in ESI†).
1H NMR titration in DMSO-d6 (Fig. 6) clearly demonstrates the interaction of NEDAP with histidine as significant spectral changes have been observed upon gradual addition of histidine to NEDAP. The imine (–CH = N–) proton at 9.36 ppm of free NEDAP has been downfield shifted to 9.41 ppm in presence of histidine. Similarly, the –NH proton at 8.986 ppm has also downfield shifted to 9.031 ppm. Although, no significant shift of aromatic protons has been observed, however, most of them behave differently in presence of histidine. This fact supports the restricted rotation of NEDAP in presence of histidine. Upon addition of histidine, the aliphatic –CH2 protons of NEDAP have up-field shifted to an extent of ∼0.05 ppm (from 4.09 ppm→ 4.04 ppm and 3.56 ppm → 3.50 ppm respectively). Interestingly, significant higher shift of histidine protons have been observed, e.g., –CH proton (δ = 8.07 ppm), in between two N-centers of imidazole unit has experienced a large up-field shift ∼1.0 ppm (δ = 7.0 ppm) while the other aromatic proton (δ = 7.12 ppm) has also up-field shifted by 0.25 ppm (δ = 6.87 ppm). All these strongly suggest the formation of inter-molecular H-bonding.
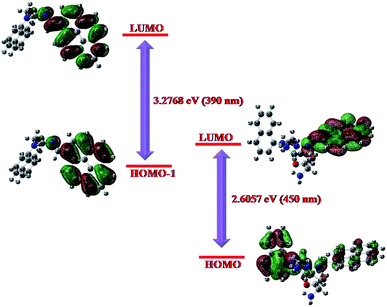
The energy optimized geometries of NEDAP and its histidine adduct are presented in Fig. 7. In the adduct, N (68)-H (44) and N (9)-H (7) distances are 1.99 Å and 1.76 Å respectively, within the range of H-bond distance. Moreover, optimized geometry of the adduct also supports the formation of molecular ring. Theoretical UV-Vis spectra of NEDAP and its histidine adduct are generated from TDDFT calculation using CPCM formalism, which considers the solvent as a polarisable continuum but not as discrete solvent molecules for solvation of the solute.32 Theoretical predictions (Fig. 8) are in close agreement to the experimentally observed peaks. The absorption peak of NEDAP at 390 nm is mainly due to the pyrene unit whereas a broad shoulder near 450 nm in the spectrum of [NEDAP–His] adduct is due to the charge transfer from naphthalene to pyrene unit.
 | ||
| Fig. 7 Energy optimized geometries of NEDAP [A] and its histidine adduct [B] using B3LYP/6-31 G basis set. | ||
Histidine treated and untreated cells are observed under fluorescence microscope in presence of NEDAP. Fig. 9 indicates that the probe can easily permeate the cell membrane of living cell tested without destroying them as the cells remain alive even after 30 minutes exposure to 1 μM NEDAP.
Conclusions
A new low cost fluorescence probe NEDAP has been synthesized by a facile one step Schiff base condensation reaction between N1-(naphthalen-1-yl)ethane-1,2-diamine hydrochloride with pyrene-1-carbaldehyde. Greater proton affinity of basic N-center of histidine allows to form a molecular ring via intermolecular H-bonding with NEDAP which imparts rigidity to the molecular assembly and inhibits the TICT process. Theoretical studies (DFT calculations) nicely support this fact substantiated from NMR titration and mass spectra. NEDAP can detect 0.01 μM histidine, below the level present in living systems (blood serium). Visible light excitation allows the probe in vivo studies, particularly intracellular histidine determination.Notes and references
- (a) J. D. Kopple and M. E. Swendseid, J. Clin. Invest., 1975, 55, 881 CrossRef CAS PubMed; (b) S. E. Snyderman, A. Boyer, E. Roitman, L. E. Holt Jr and P. H. Prose, Pediatrics, 1963, 31, 786 CAS.
- T. E. Creighton, Encyclopedia of Molecular Biology, Wiley, New York, 1999, vol. 2, p. 1147 Search PubMed.
- G. N. Chen, X. P. Wu, J. P. Duan and H. Q. Chen, Talanta, 1999, 49, 319 CrossRef.
- Y. He, X. Wang, J. Zhu, S. Zhong and G. Song, Analyst, 2012, 137, 4005 RSC.
- M. L. Rao, H. Stefan, C. Scheid, A. D. S. Kuttler and W. Froscher, Epilepsia, 1993, 34, 347 CrossRef CAS.
- P. M. Kovach and M. E. Meyerhoff, Anal. Chem., 1982, 54, 217 CrossRef CAS.
- L. Zhou, N. Yan, H. G. Zhang, X. M. Zhou, Q. S. Pu and Z. I. Hu, Talanta, 2010, 82, 72 CrossRef CAS PubMed.
- J. Meng, W. Zhang, C. X. Cao, L. Y. Fan, J. Wu and Q. L. Wang, Analyst, 2010, 135, 1592 RSC.
- N. Tateda, K. Matsuhisa, K. Hasebe and T. Miura, Anal. Sci., 2001, 17, 775 CrossRef CAS.
- S. Wadud, M. M. Or-Rashid and R. Onodera, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2002, 767, 369 CrossRef CAS.
- M. Shahlaei, M. B. Gholivand and A. Pourhossein, Electroanalysis, 2009, 21, 2499 CAS.
- M. A. Hortalá, L. Fabbrizzi, N. Marcotte, F. Stomeo and A. Taglietti, J. Am. Chem. Soc., 2003, 125, 20 CrossRef PubMed.
- S. Y. Zhang, C. M. Yang, W. P. Zhu, B. B. Zeng, Y. J. Yang, Y. F. Xu and X. H. Qian, Org. Biomol. Chem., 2012, 10, 1653 CAS.
- F. Pu, Z. Z. Huang, J. S. Ren and X. G. Qu, Anal. Chem., 2010, 82, 8211 CrossRef CAS PubMed.
- Z. Huang, J. Du, J. Zhang, X. Q. Yu and L. Pu, Chem. Commun., 2012, 48, 3412 RSC.
- L. Fabbrizzi, G. Francese, M. Licchelli, A. Perotti and A. Taglietti, Chem. Commun., 1997, 581 RSC.
- M. A. Hortala, L. Fabbrizzi, N. Marcotte, F. Stomeo and A. Taglietti, J. Am. Chem. Soc., 2003, 125, 20 CrossRef CAS PubMed.
- (a) J. F. Folmer-Andersen, V. M. Lynch and E. V. Anslyn, Chem.–Eur. J., 2005, 11, 5319 CrossRef CAS PubMed; (b) S.-K. Sun, K.-X. Tu and X.-P. Yan, Analyst, 2012, 137, 2124 RSC.
- G. Patel and S. Menon, Chem. Commun., 2009, 3563 RSC.
- R. Yang, K. Wang, L. Long, D. Xiao, X. Yang and W. Tan, Anal. Chem., 2002, 74, 1088 CrossRef CAS.
- (a) Z. Huang, J. Du, J. Zhang, X.-Q. Yu and L. Pu, Chem. Commun., 2012, 48, 3412 RSC; (b) J. Du, Z. Huang, X.-Q. Yu and L. Pu, Chem. Commun., 2013, 49, 5399 RSC.
- E. Oliveira, C. Santos, P. Poeta, J. L. Capelo and C. Lodeiro, Analyst, 2013, 138, 3642 RSC.
- (a) A. Banerjee, A. Sahana, S. Guha, S. Lohar, I. Hauli, S. K. Mukhopadhyay, J. S. Matalobos and D. Das, Inorg. Chem., 2012, 51, 5699 CrossRef CAS PubMed; (b) A. Banerjee, D. Karak, A. Sahana, S. Guha, S. Lohar and D. Das, J. Hazard. Mater., 2011, 186, 738 CrossRef CAS PubMed; (c) A. Sahana, A. Banerjee, S. Das, S. Lohar, D. Karak, B. Sarkar, S. K. Mukhopadhyay, A. K. Mukherjee and D. Das, Org. Biomol. Chem., 2011, 9, 5523 RSC; (d) A. Sahana, A. Banerjee, S. Lohar, S. Guha, S. Das, S. K. Mukhopadhyay and D. Das, Analyst, 2012, 137, 3910 RSC; (e) S. Das, A. Sahana, A. Banerjee, S. Lohar, D. A. Safin, M. G. Babashkina, M. Bolte, Y. Garcia, I. Hauli, S. K. Mukhopadhyay and D. Das, Dalton Trans., 2013, 42, 4757 RSC; (f) A. Sahana, A. Banerjee, S. Lohar, B. Sarkar, S. K. Mukhopadhyay and D. Das, Inorg. Chem., 2013, 52, 3627 CrossRef CAS PubMed.
- (a) A. Banerjee, A. Sahana, S. Lohar, I. Hauli, S. K. Mukhopadhyay, D. A. Safin, M. G. Babashkina, M. Bolte, Y. Garcia and D. Das, Chem. Commun., 2013, 49, 2527 RSC; (b) A. Sahana, A. Banerjee, S. Guha, S. Lohar, A. Chattopadhyay, S. K. Mukhopadhyay and D. Das, Analyst, 2012, 137, 1544 RSC; (c) S. Lohar, A. Sahana, A. Banerjee, A. Banik, S. K. Mukhopadhyay, J. S. Matalobos and D. Das, Anal. Chem., 2013, 85, 1778 CrossRef CAS PubMed; (d) A. Sahana, A. Banerjee, S. Lohar, S. Panja, S. K. Mukhopadhyay, J. S. Matalobos and D. Das, Chem. Commun., 2013, 49, 7231 RSC; (e) A. Sahana, A. Banerjee, S. Lohar, A. Chottapadhyay, S. K. Mukhopadhyay and D. Das, RSC Adv., 2013, 3, 14044 RSC; (f) A. Banerjee, A. Sahana, S. Lohar, S. Panja, S. K. Mukhopadhyay and D. Das, RSC Adv., 2014, 4, 3887, 10.1039/c3ra45362f.
- S. Das, S. Guha, A. Banerjee, S. Lohar, A. Sahana and D. Das, Org. Biomol. Chem., 2011, 9, 7097 CAS.
- A. Banerjee, A. Sahana, S. Lohar, B. Sarkar, S. K. Mukhopadhyay and D. Das, RSC Adv., 2013, 3, 14397 RSC.
- BRS Biochemistry, Molecular Biology and Genetics, (Fifth edition): T. A. Swanson, S. I. Kim, M. J. Glucksman Search PubMed.
- (a) S. Das, A. Sahana, A. Banerjee, S. Lohar, S. Guha, J. S. Matalobos and D. Das, Anal. Methods, 2012, 4, 2254 RSC; (b) H. Sharma, K. Narang, N. Singh and N. Kaur, Mater. Lett., 2012, 84 Search PubMed; (c) R. Azadbakht and J. Khanabadi, Inorg. Chem. Commun., 2013, 30, 21 CrossRef CAS PubMed; (d) U. Heinz, K. Hegetschweiler, P. Acklin, B. Faller, R. Lattmann and H. P. Schnebli, Angew. Chem., Int. Ed., 1999, 38, 2568 CrossRef CAS.
- (a) E. Lippert, W. Luder and H. Boos, in Advances in Molecular Spectroscopy, ed. A. Mangini, Pergamon, Oxford, 1962, p. 443 Search PubMed; (b) K. Rotkiewicz, K. H. Grellmann and Z. R. Grabowski, Chem. Phys. Lett., 1973, 19, 315 CrossRef CAS; (c) K. A. Zachariasse, M. Grobye and E. Tauer, Chem. Phys. Lett., 1997, 274, 372 CrossRef CAS; (d) W. Schuddeboom, S. A. Jonker and J. M. Warman, J. Phys. Chem., 1992, 96, 10809 CrossRef CAS; (e) A. L. Sobolewski, W. Sudholt and W. Domcke, J. Phys. Chem. A, 1998, 102, 2716 CrossRef CAS; (f) D. I. Schuster, M. D. Goldstein and P. Bane, J. Am. Chem. Soc., 1977, 99, 187 CrossRef CAS; (g) L. C. T. Soute, Chem. Phys. Lett., 1992, 195, 255 CrossRef; (h) P. R. Callis and R. W. Wilson, Chem. Phys. Lett., 1972, 13, 417 CrossRef CAS; (i) M. Glasbeek and H. Zhang, Chem. Rev., 2004, 104, 1929 CrossRef CAS PubMed; (j) J. A. Mondal, H. N. Ghosh, T. K. Ghanty, T. Mukherjee and D. K. Palit, J. Phys. Chem. A, 2006, 110, 3432 CrossRef CAS PubMed.
- (a) H. A. Benesi and J. H. Hildebrand, J. Am. Chem. Soc., 1949, 71, 2703 CrossRef CAS; (b) A. Banerjee, A. Sahana, S. Das, S. Lohar, S. Guha, B. Sarkar, S. K. Mukhopadhyay, A. K. Mukherjee and D. Das, Analyst, 2012, 137, 2166 RSC.
- T. Biver, M. Pulzonetti, F. Secco, M. Venturini and S. Yarmoluk, Biochem. Biophys., 2006, 451, 103 CrossRef CAS PubMed.
- (a) S. Miertus, E. Scrocco and J. Tomasi, Chem. Phys., 1981, 55, 117 CrossRef CAS; (b) M. Cossi, V. Barone, R. Cammi and J. Tomasi, Chem. Phys. Lett., 1996, 255, 327 CrossRef CAS; (c) M. Cossi, V. Barone and M. A. Robb, J. Chem. Phys., 1999, 111, 5295 CrossRef CAS PubMed; (d) M. Cossi, G. Scalmani, N. Rega and V. Barone, J. Chem. Phys., 2002, 117, 43 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra46604c |
| This journal is © The Royal Society of Chemistry 2014 |