Multi-walled carbon nanotube supported Fe3O4NPs: an efficient and reusable catalyst for the one-pot synthesis of 4H-pyran derivatives†
Abdollah Fallah-Shojaei*,
Khalil Tabatabaeian,
Farhad Shirini and
Seyyedeh Zoha Hejazi
Department of Chemistry, College of Science, University of Guilan, PO Box 41335–1914, Rasht, Iran. E-mail: a.f.shojaie@guilan.ac.ir; Fax: +98 1313233262; Tel: +98 1313233262
First published on 10th December 2013
Abstract
Multi-walled carbon nanotube supported Fe3O4 nanoparticles have been prepared and characterized by Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), vibrating sample magnetometry (VSM), and transmission and scanning electron microscopy (TEM and SEM). This superparamagnetic nanocomposite, which could be conveniently separated by using an external magnet, can be used as an efficient catalyst for the promotion of the synthesis of 4H-pyran derivatives via a one-pot multicomponent reaction of an aldehyde, malononitrile and β-diketones. Some of the advantages of this work are that it is environmentally benign, has an easy work-up procedure, saves energy and uses mild reaction conditions.
1 Introduction
In recent years, nanotechnology has rapidly progressed in chemistry, physics, materials science and biotechnology to create magnetic nanoparticles (MNPs) that have unique physical and chemical properties such as a large surface area to act either as heterogeneous promoters for catalytic reactions or as a support for homogeneous catalysts.1,2 MNPs are commonly composed of magnetic elements such as iron, nickel, cobalt and their oxides.3 Among them, Fe3O4 MNPs have attracted much attention because of their inherent properties such as their fast response under an applied external magnetic field, high coercivity, environmental friendliness and high surface area per weight, which causes their higher reactivity, greater selectivity, operational simplicity, non-corrosive nature, moisture insensitivity, low toxicity, superparamagnetic behavior and ease of separation from the reaction medium by using external magnets, which minimizes the loss of catalyst during separation.4–9However, due to their large specific surface area and high surface energy, MNPs tend to aggregate and exhibit negligible dispersion in water and organic solvents.10,11 Furthermore, Fe3O4 contains Fe2+ which is easily oxidized, thus decreasing the magnetism of MNPs.12 In order to enhance the dispersion and oxidation resistance of MNPs, adjustment and a surface coating of Fe3O4 MNPs is essential.
Nowadays, graphitized carbon structures, such as multi-walled carbon nanotubes (MWCNTs) are receiving more attention due to their exceptional mechanical, thermal and electrical properties. Decoration of the external surface of the CNTs provides an effective barrier against oxidation and acid erosion. These facts indicate that it is possible to synthesize carbon-coated magnetic nanoparticles, which are thermally stable and have a high stability against oxidation and acid leaching, which is crucial for some applications.13 CNTs loaded with Fe3O4 nanoparticles on their external surface show an exceptional performance and have potential applications in various fields such as catalysis,14,15 reinforcing materials,16 electrodes,17 and wastewater treatment.18 Also, Fe3O4-supported catalysts can be easily separated from the reaction mixture by an external permanent magnet.19–22
The synthesis of 4H-pyran and its derivatives has attracted much attention from synthetic chemists due to their useful biological and pharmacological properties. Most of them can be used as anticoagulant, anticancer, diuretic, spasmolytic and antianaphylactic agents.23,24 A series of natural products contains substituted 4H-pyrans in their structural units.25
A variety of reagents such as sodium bromide,26 hexadecyldimethyl benzyl ammonium bromide,27 tetramethyl ammonium hydroxide,28 sodium selenate,29 iodine,30 tetrabutylammonium bromide,31 cerium(III) chloride,32 lithium bromide,33 Amberlite IRA-40 (OH−),34 acidic ionic liquids,35 L-proline,36 nano-ZnO,37 nano-SiO2,38 phenylboronic acid,39 triethylenetetraammoniumtrifluoroacetate [TETA]TFA,40 ZnO-beta zeolite,41 trisodium citrate,42 basic ionic liquids,43 high surface area MgO,44,45 nanosized MgO,46 solid acids,47,48 and organic solvents such as DMF and acetic acid,49,50 have been used to catalyze these reactions. Pyrans have also been synthesized under microwave51 and ultrasound52 irradiation. Recently, some two-component53 and three-component54,55 condensations have been introduced for the synthesis of 4H-chromenes.
However, each of the above methods suffers from disadvantages such as low yields, unavailability or toxicity of the reagents, long reaction times, hazardous organic solvents, vigorous reaction conditions and a tedious work-up. Therefore, to overcome these drawbacks, there is still a great demand to develop a simple and efficient catalytic system for the synthesis of pyrans.
With regard to the importance of this topic and due to the advantages of multicomponent reactions (MCRs) such as atom economy, high selectivity and greater efficiency, we decided to demonstrate a simple, rapid and effective one-pot MCR for the synthesis of 4H-pyran derivatives using Fe3O4NPs/MWCNTs as a supermagnetic and reusable catalyst. To the best of our knowledge, our work is the first report using a catalytic amount of this reagent to catalyze the synthesis of 4H-pyran derivatives.
2 Results and discussions
2.1 Catalyst characterization
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch mode of the carboxylic acid in the CNTs. Other peaks at 1133 and 1165 cm−1 are attributed to the stretch bonds of the phenyl-carbonyl C–C and a strong peak at around 565 cm−1 is related to the Fe–O–Fe interactions in Fe3O4.
O stretch mode of the carboxylic acid in the CNTs. Other peaks at 1133 and 1165 cm−1 are attributed to the stretch bonds of the phenyl-carbonyl C–C and a strong peak at around 565 cm−1 is related to the Fe–O–Fe interactions in Fe3O4.
Moreover, peaks at 1358, 2847 and 2917 cm−1 are ascribed to the in-plane bending vibration of the C–H stretch vibration originating from the surface of tubes.
After preparation and characterization of Fe3O4 NPs/MWCNTs, the catalytic activity of this catalyst was examined in a three component reaction between aromatic aldehydes, malononitrile, and 1,3-diketones. Initially, 4-chlorobenzaldehyde, malononitrile and dimedone in an equimolar ratio were employed as the model reactants and the effect of solvent, catalyst and temperature was investigated in the presence of various amounts of Fe3O4 NPs/MWCNTs (2 to 10 mg) in different solvents at temperatures ranging from 25 °C to reflux (Table 1) (Scheme 1).
| Entry | Fe3O4 NPs/MWCNTs (mg) | Solvent | Temp. (°C) | Time (min) | Yieldb (%) |
|---|---|---|---|---|---|
| a All reactions were run with 4-chlorobenzaldehyde (1.0 mmol), dimedone (1.0 mmol), and malononitrile (1.0 mmol).b Isolated yields.c Pure Fe3O4 NPs is used as a catalyst. | |||||
| 1 | 10 | Water | Reflux | 60 | 61 |
| 2 | 10 | n-Hexane | Reflux | 180 | — |
| 3 | 10 | Acetonitrile | Reflux | 60 | 31 |
| 4 | 10 | Ethanol | Reflux | 10 | 94 |
| 5 | 10c | Ethanol | Reflux | 30 | 78 |
| 6 | 7 | Ethanol | Reflux | 10 | 95 |
| 7 | 5 | Ethanol | Reflux | 8 | 97 |
| 8 | 3 | Ethanol | Reflux | 60 | 82 |
| 9 | — | Ethanol | Reflux | 180 | Trace |
| 10 | 5 | None | 80 | 60 | Trace |
| 11 | 5 | Ethanol | r.t. | 180 | Trace |
| 12 | 5 | Ethanol | 50 | 60 | 52 |
 | ||
| Scheme 1 Synthesis of 2-amino-4-(4-chlorophenyl)-3-cyano-7,7-dimethyl-5-oxo-4H-5,6,7,8 tetrahydrobenzo[b]pyran as a model reaction. | ||
The reaction was carried out in the presence of several solvents such as EtOH, MeCN, n-hexane, H2O and also in the absence of solvent using 10 mg of catalyst. As shown in Table 1, the best result was obtained when the reaction was carried out in EtOH (Table 1, entry 4). Then, the effect of catalyst loading was studied and condensation of reactants in an equimolar ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was performed in the presence of various amounts of the catalyst under reflux conditions. No product was observed in the absence of the catalyst. The yields of the product (Table 1) indicate that 5 mg of Fe3O4 NPs/MWCNTs is the optimum amount of the catalyst for this reaction (Table 1, entry 6). The use of higher amounts of the catalyst neither improved the yield nor the reaction time. The effect of temperature on the reaction was monitored and reflux conditions were chosen for all further reactions. No reaction was detected after stirring the reaction mixture at room temperature for 180 min.
1) was performed in the presence of various amounts of the catalyst under reflux conditions. No product was observed in the absence of the catalyst. The yields of the product (Table 1) indicate that 5 mg of Fe3O4 NPs/MWCNTs is the optimum amount of the catalyst for this reaction (Table 1, entry 6). The use of higher amounts of the catalyst neither improved the yield nor the reaction time. The effect of temperature on the reaction was monitored and reflux conditions were chosen for all further reactions. No reaction was detected after stirring the reaction mixture at room temperature for 180 min.
Furthermore, in order to show the excellent catalytic activity of this catalyst, we carried out the synthesis of the model reaction catalyzed by pure Fe3O4 NPs under the same reaction conditions. It was shown that the yield of the desired product in the presence of the pure Fe3O4 NPs was lower than that in the presence of Fe3O4 NPs/MWCNTs.
This could be due to the fact that Fe3O4 nanoparticles tend to aggregate, because of their high surface area to volume ratio and the strong dipole–dipole attraction between the particles.10,11 Also, the magnetic properties of nano-Fe3O4 decrease upon the oxidation of Fe2+in the catalyst structure.12
According to the outcomes in Table 1, it can be concluded that the best yield of the products was achieved by using the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of reactants and 5 mg of Fe3O4 NPs/MWCNTs, under reflux conditions in EtOH.
1 ratio of reactants and 5 mg of Fe3O4 NPs/MWCNTs, under reflux conditions in EtOH.
After optimization, the applicability of the method was studied by synthesis of several types of substituted 4H-pyrans using a series of aldehydes (Table 2) (Scheme 2). As can be seen in Table 2, the aromatic aldehydes with different substituents at the ortho-, meta- or para-positions were examined and in all cases gave corresponding products in good to excellent yields. Notably, the aromatic aldehydes with electron-withdrawing groups (such as nitro and halide) reacted faster with slightly improved yields than their electron donating counterparts (such as hydroxyl and methoxy). The pure products were identified by their melting points, IR, 1H and 13C NMR, which are in agreement with those reported in the literature.
| Entry | R | Productb | MW | Reflux | MP (°C) | |||
|---|---|---|---|---|---|---|---|---|
| Time (s) | Yieldc (%) | Time (min) | Yieldc (%) | Found | Reported [References] | |||
| a Reaction conditions: aldehyde (1 mmol), malononitrile (1 mmol), cyclic-1,3-diketones (1 mmol), catalyst (5 mg), ethanol (5 mL), reflux or MW.b Melting points, IR, 1H NMR and 13C NMR were in accordance with those of authentic samples.c Isolated yield. | ||||||||
| 1 | 4-Chloro | 1a | 25 | 98 | 8 | 97 | 212–214 | 212–214 [38] |
| 2 | 3-Nitro | 2a | 40 | 96 | 10 | 95 | 209–210 | 210–211 [38] |
| 3 | 4-Cyano | 3a | 20 | 97 | 5 | 98 | 224–227 | 224–226 [58] |
| 4 | 2-Chloro-6-fluoro | 4a | 20 | 97 | 8 | 95 | 202–204 | — |
| 5 | 2,4-Dichloro | 5a | 20 | 96 | 7 | 95 | 119–122 | 114–116 [40] |
| 6 | 4-Nitro | 6a | 30 | 96 | 8 | 96 | 176–178 | 177–178 [38] |
| 7 | 4-Methoxy | 7a | 90 | 87 | 20 | 85 | 197–200 | 198–200 [38] |
| 8 | 3-Phenoxy | 8a | 180 | 85 | 40 | 87 | 181–183 | 182–184 [38] |
| 9 | 4-Isopropyl | 9a | 300 | 86 | 50 | 84 | 199–201 | 198–200 [38] |
| 10 | 4-Nitro | 1b | 60 | 91 | 15 | 90 | 237–239 | 240–241 [39] |
| 11 | 2-Chloro-6-fluoro | 2b | 40 | 92 | 10 | 90 | 228–230 | — |
| 12 | 2,4-Dichloro | 3b | 35 | 90 | 8 | 88 | 221–223 | 222–224 [40] |
| 13 | 4-Chloro | 4b | 120 | 89 | 20 | 87 | 227–229 | 229–230 [39] |
| 14 | 4-Methoxy | 5b | 180 | 81 | 22 | 80 | 197–199 | 198–200 [39] |
In order to develop the scope of this method, we found that when ethyl acetoacetate was used instead of a cyclic-1,3-diketone (e.g., dimedone or 1,3-cyclohexanedione) under the same reaction conditions, 6-amino-5-cyano-2-methyl-4-aryl-4H-pyran-3-carboxylic acid ethyl ester derivatives were prepared in high yields and short reaction times (Scheme 3). The results are presented in Table 3.
 | ||
| Scheme 3 Fe3O4 NPs/MWCNTs catalyzed synthesis of 6-amino-5-cyano-2-methyl-4-aryl-4H-pyran-3-carboxylic acid ethyl ester derivatives. | ||
| Entry | R | Productb | MW | Reflux | MP (°C) | |||
|---|---|---|---|---|---|---|---|---|
| Time (min) | Yieldc (%) | Time (min) | Yieldc (%) | Found | Reported [References] | |||
| a Reaction conditions: aldehyde (1 mmol), malononitrile (1 mmol), ethyl acetoacetate (1 mmol), catalyst (5 mg), ethanol (5 mL), reflux or MW (40 °C).b Melting points, IR, 1H NMR and 13C NMR were in accordance with those of authentic samples.c Isolated yield. | ||||||||
| 1 | 3-Nitro | 1c | 3 | 90 | 25 | 89 | 173–175 | 171–173 [38] |
| 2 | 3,4-Difluoro | 2c | 1.5 | 94 | 15 | 93 | 166–168 | — |
| 3 | 4-Methoxy | 3c | 6 | 83 | 50 | 82 | 137–140 | 137–139 [38] |
| 4 | Phenyl | 4c | 4 | 83 | 35 | 80 | 113–115 | 112–114 [38] |
| 5 | 4-Chloro | 5c | 2 | 93 | 20 | 92 | 174–176 | 175–177 [38] |
| 6 | 4-Nitro | 6c | 2 | 92 | 20 | 90 | 176–177 | 176–178 [38] |
Due to the benefits of microwave-assisted reactions such as high product yields, a faster and cleaner approach and an environmentally benign source of energy, we investigated the effect of MW irradiation on the synthesis of target compounds. After optimization of the reaction conditions, using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of reactants, 3 mg of Fe3O4 NPs/MWCNTs as the catalyst, 3 mL ethanol as the solvent and MW irradiation at a power level of 40% at 40 °C, we examined the generality of the reaction. As expected in all cases, enhancement in the reaction speed and reaction times were observed (Tables 2 and 3). It seems that the absorbing characteristics of carbon nanotubes could improve the reaction time and the yield of products.59–61
1 ratio of reactants, 3 mg of Fe3O4 NPs/MWCNTs as the catalyst, 3 mL ethanol as the solvent and MW irradiation at a power level of 40% at 40 °C, we examined the generality of the reaction. As expected in all cases, enhancement in the reaction speed and reaction times were observed (Tables 2 and 3). It seems that the absorbing characteristics of carbon nanotubes could improve the reaction time and the yield of products.59–61
Furthermore, the efficiency of this catalyst was demonstrated by the comparison between different catalysts and the synthesis of 4H-pyran (1a) was considered as a representative example. It is noteworthy that the reaction time and the yield in the present method is better in comparison with those of others (Table 4).
| Entry | Catalyst amount | Solvent | Time (min) | Yieldb (%) | References |
|---|---|---|---|---|---|
| a All reactions were run with 4-chlorobenzaldehyde (1.0 mmol), dimedone (1.0 mmol), and malononitrile (1.0 mmol) at reflux conditions.b Isolated yield. | |||||
| 1 | ZrO2 (200 mg) | EtOH | 90 | 20 | [62] |
| 2 | MgO (200 mg) | EtOH | 85 | 45 | [62] |
| 3 | [TETA]TFA (0.1 mmol) | EtOH | 30 | 93 | [40] |
| 4 | ZnO (3 mg) | EtOH | 10 | 80 | [37] |
| 5 | PhB(OH)2 (5 mol%) | H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH (1 EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
30 | 84 | [39] |
| 6 | Na2SeO4 (100 mg) | H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH (1 EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
180 | 90 | [29] |
| 7 | Fe3O4 NPs/MWCNTs (5 mg) | EtOH | 8 | 97 | — |
Finally, the recycling performance of the catalyst in the model reaction was studied. For this reason, the catalyst was separated by use of an external magnet. The magnetic Fe3O4NPs/MWCNTs were washed three to four times with water and ethanol and dried at room temperature for 5 h. Fig. 5 shows that this catalyst could be used five times with only a slight decrease in its catalytic activity. The stability of the catalyst is confirmed via determination of Fe ion leaching by atomic absorption spectroscopy. Trace metal ions were detected in the filtrate of this reaction.
 | ||
| Fig. 5 Reusability of Fe3O4 NPs/MWCNTs in the model reaction (Table 1, entry 7). | ||
A possible mechanism for the synthesis of 4H-pyran derivatives has been proposed in Scheme 4. Based on this mechanism, Fe3O4 NPs/MWCNTs is an effective catalyst for the formation of cyanoolefin A which is the Knoevenagel condensation product of arylaldehyde 1 and the active anion of malononitrile compound 2. Dimedone, after proton transfer and tautomerization, is in its enolic form, which in the presence of Fe3O4 NPs/MWCNTs could easily react with cyanoolefin A, resulting in the formation of intermediate B which converts to product 3.
In summary, this is the first report of the synthesis of substituted 4H-chromene derivatives catalyzed by magnetic Fe3O4 NPs/MWCNTs as a novel, effective and easily reusable catalyst, which provided excellent yields (80–97%) in short reaction times.
3 Experimental
3.1 General
All materials and solvents were purchased from Merck and Fluka, and used without further purification. Yields refer to the isolated products. Products were characterized by their physical constants, comparison with authentic samples, and IR and NMR spectroscopy.3.2 Instrumentation
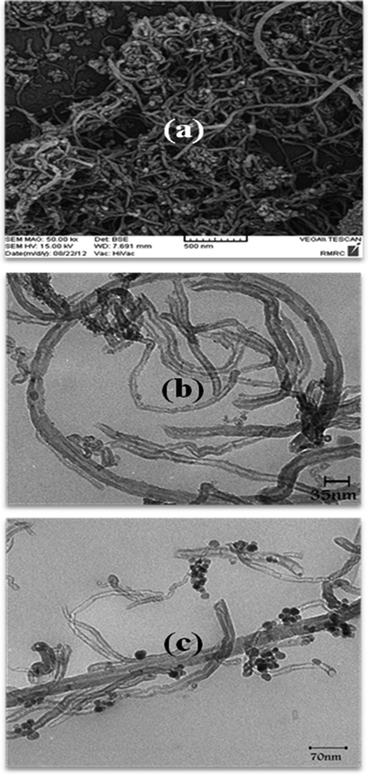
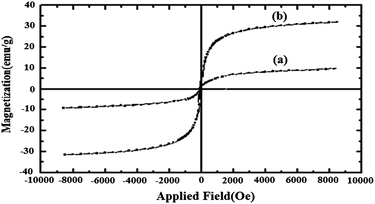
IR spectra were obtained with KBr discs on a Perkin-Elmer model Spectrum one FT-IR Spectrometer. The structure of the synthesized catalysts was characterized by X-ray diffraction (XRD-Equinox-3000, INEL, France). The XRD patterns were obtained using Cu Kα radiation (the wavelength was 1.54056 Å) at a current of 200 mA and a voltage of 40 kV in the 2θ range of 10–100 with a scanning rate of 8° per min. The surface microscopic morphology of MWCNTs and Fe3O4 NP decorated MWCNTs was visualized by a scanning electron microscope (SEM-MIRAII TESCAN). The size of the Fe3O4 NPs was investigated by a transmission electron microscope (TEM-PHILIPS MC 10) with an acceleration voltage of 80 kV. Magnetic study was performed by a vibrating sample magnetometer at room temperature (VSM JDM-13). A Perkin Elmer Analyst 100 Atomic Absorption Spectrophotometer was used. The reaction progress was checked by thin-layer chromatography (TLC) with detection by UV light. 1H NMR spectra were obtained on a Bruker DRX-400 Avance spectrometer and 13C NMR spectra were obtained on a Bruker DRX-100 Avance spectrometer. Samples were analyzed in DMSO-d6, and chemical shift values are reported in ppm relative to tetramethylsilane (TMS) as the internal reference. Melting points were measured on an electrothermal apparatus. Elemental analyses were made by a Carlo-Erba EA1110 CNNO-S analyzer and agreed with the calculated values. Microwave experiments were conducted in a Milestone Microwave (Microwave Labstation-MLS GmbH-ATC-FO 300) apparatus.3.3 Preparation of the catalyst (Fe3O4 NPs/MWCNTs)
Firstly, 96 mg of functionalized MWCNTs (carboxylic acid functionalized MWCNTs with a purity of more than 95% were purified by using sulfuric acid and a heat treatment method) were well dispersed in 30 mL of distilled water in a two necked round bottom flask (100 mL) by ultrasonic irradiation for 30 min. After adding 81 mg of FeCl3·6H2O, the solution was stirred vigorously for 30 min. Afterward, 120 mg FeCl2·4H2O was slowly added into the mixture under stirring for 30 min. The whole process was performed under Argon atmosphere. Then 8 mL of concentrated aqueous solution of NH3 was added into the solution drop wise for 1 h. Thereafter, the mixture was stirred at 60 °C for 2 h. After cooling the solution to room temperature, the black magnetic Fe3O4NPs/MWCNTs were centrifuged at 6000 rpm and rinsed several times with deionized water and dried at 100 °C for 12 h. Measuring the amount of residual Fe ions in the filtrate by spectrophotometry showed that more than 95% of the iron is stabilized on the surface of the MWCNTs. Scheme 5 illustrates the preparation process of the Fe3O4 nanoparticles decorated on the outer surface of functionalized MWCNTs. As can be seen, positive ferrous and ferric ions are in close proximity with the carboxylic groups of the MWCNTs and they are converted to Fe3O4 NPs after the addition of a concentrated aqueous solution of NH3 into the solution drop by drop.3.4 General procedure for the synthesis of 4H-pyran catalyzed by Fe3O4 NPs/MWCNTs
Fe3O4 NPs/MWCNTs (5 mg) was added to a mixture of aldehyde (1.0 mmol), malononitrile (1.0 mmol), and cyclic 1,3-diketone or ethyl acetoacetate (1.0 mmol) in ethanol (5 mL). The reaction mixture was stirred magnetically under refluxing conditions for the appropriate time. In the case of the microwave assisted reactions, the mixture was irradiated at 800 W at 40 °C for a few minutes depending on the reactants. After completion of the reaction as indicated by TLC, the catalyst was collected by magnetic separation using an external magnet and washed repeatedly with warm ethanol. The aqueous phase was filtered and cooled to room temperature. Then, the solid product was collected and washed with warm ethanol to afford the pure product. For further purification, products were recrystallized from ethanol.Except for some compounds (Table 2, entries 4, 11 and Table 3, entry 2), all products are known compounds. The spectroscopic and physical data for all known compounds were found to be identical to those described in the literature.
The spectral (IR, 1H and 13C NMR) data of the new compounds are presented below:
4 Conclusions
In conclusion, we have reported an easy, convenient and novel method for the synthesis of a series of biologically and pharmacologically active 4H-pyran derivatives catalyzed by Fe3O4 NPs/MWCNTs using a three-component condensation in ethanol as a green solvent. In this regard, we have prepared the catalyst and confirmed the nanostructure of the Fe3O4 NPs on the surface of the MWNTs by XRD, SEM and TEM images. We found that Fe3O4 NPs/MWCNTs can be easily separated from the reaction mixture by an external permanent magnet. The key advantages of this procedure include high yields, operational simplicity, a reusable catalyst, a non-toxic solvent, short reaction times, minimum pollution of the environment and purification of the compounds by nonchromatographic methods which makes it a useful and attractive process for the preparation of the desired compounds.Acknowledgements
The authors are grateful to the Research Council of the University of Guilan for partial support of this study.Notes and references
- A. Gißibl, PhD dissertation, Regensburg, 2006.
- S. Jain and O. Reiser, ChemSusChem, 2001, 1, 534 CrossRef PubMed.
- T. K. Indira and P. K. Lakshmi, Int. J. Pharm. Sci. Nanotechnol., 2010, 3, 1035 CAS.
- (a) P. Riente, C. Mendoza and M. A. Pericas, J. Mater. Chem., 2011, 21, 7350 RSC; (b) K. V. S. Ranganath, J. Kloesges, A. H. Schafer and F. Glorius, Angew. Chem., Int. Ed. Eng., 2010, 49, 7786 CrossRef CAS PubMed; (c) B. Karami, S. J. Hoseini, S. Nikoseresht and S. Khodabakhshi, Chin. Chem. Lett., 2012, 23, 173 CrossRef CAS PubMed; (d) B. Karami, S. Nikoseresht and S. Khodabakhshi, Chin. J. Catal., 2012, 33, 298 CrossRef CAS.
- B. Karami, S. J. Hoseini, K. Eskandari, A. Ghasemi and H. Nasrabadi, Catal. Sci. Technol., 2012, 2, 331 CAS.
- A. L. Morel, S. I. Nikitenko, K. Gionnet, A. Wattiaux, J. Lai-Kee-Him, C. Labrugere, B. Chevalier, G. Deleris, C. Petibois, A. Brisson and M. Simonoff, J. Am. Chem. Soc., 2008, 2, 847 CAS.
- Y. Weia, B. Hanb, X. Hua, Y. Linc, X. Wangd and X. Denga, Procedia Eng., 2012, 27, 632 CrossRef PubMed.
- Y. S. Kim and Y. H. Kim, J. Magn. Magn. Mater., 2003, 267, 105 CrossRef CAS.
- M. Nasr-Esfahani, S. J. Hoseyni and F. Mohammadi, Chin. J. Catal., 2011, 23, 1484 CrossRef.
- Z. W. Li and Y. F. Zhu, Appl. Surf. Sci., 2003, 211, 315 CrossRef CAS.
- G.-S. Lai, H.-L. Zhang and D.-Y. Han, Sens. Actuators, B, 2008, 129, 497 CrossRef CAS PubMed.
- R. Y. Hong, J. H. Li, Sh. Zh. Zhang, H. Zh. Li, Y. Zheng, J. M. Ding and D. G. Wei, Appl. Surf. Sci., 2009, 255, 3485 CrossRef CAS PubMed.
- L. C. Varanda, M. J. Junior, W. B. Junior, Magnetic and Multifunctional Magnetic Nanoparticles in Nanomedicine: Challenges and Trends in Synthesis and Surface Engineering for Diagnostic and Therapy Applications, Biomedical Engineering, Trends in Materials Science, InTech, 2011, pp. 397–425 Search PubMed.
- B. Nigrovski, U. Zavyalova, P. Scholz, K. Pollok, M. Muller and B. Ondruschka, Carbon, 2008, 46, 1678 CrossRef CAS PubMed.
- S. Q. Song, R. C. Rao, H. X. Yang, H. D. Liu and A. M. Zhang, Nanotechnology, 2010, 21, 1 Search PubMed.
- J. T. Feng, J. H. Sui, W. Cai, J. Q. Wan, A. N. Chakoli and Z. Y. Gao, Mater. Sci. Eng., B, 2008, 150, 208 CrossRef CAS PubMed.
- Y. He, L. Huang, J. S. Cai, X. M. Zheng and S. G. Sun, Electrochim. Acta, 2010, 55, 1140 CrossRef CAS PubMed.
- C. K. Dong, X. Li, Y. Zhang, J. Y. Qi and Y. F. Yuan, Chem. Res. Chin. Univ., 2009, 25, 936 CAS.
- A. Hu, G. T. Yee and W. Lin, J. Am. Chem. Soc., 2005, 127, 12486 CrossRef CAS PubMed.
- S. Shylesh, V. Schunemann and W. R. Thiel, Angew. Chem., Int. Ed., 2010, 49, 3428 CrossRef CAS PubMed.
- K. V. S. Ranganath and F. Glorius, Catal. Sci. Technol., 2011, 1, 13 CAS.
- V. Polshettiwar, R. Luque, A. Fihri, H. Zhu, M. Bouhrara and J. M. Basset, Chem. Rev., 2011, 111, 3036 CrossRef CAS PubMed.
- L. Bonsignore, G. Loy, D. Secci and A. Calignano, J. Org. Chem., 1993, 28, 517 CAS.
- L. L. Andreani and E. Lepi, Bull. Chim. Farm., 1960, 99, 583 Search PubMed.
- S. Hatakeyama, N. Ochi, H. Numata and S. Takano, J. Chem. Soc., Chem. Commun., 1988, 1202 RSC.
- I. Devi and P. J. Bhuyan, Tetrahedron Lett., 2004, 45, 8625 CrossRef CAS PubMed.
- T. S. Jin, A. Q. Wang, F. Shi, L. S. Han, L. B. Liu and T. S. Li, ARKIVOC, 2006, xiv, 78 CrossRef.
- S. Balalaie, M. Shiekh-Ahmadi and M. Barazjanian, Catal. Commun., 2007, 8, 1724 CrossRef CAS PubMed.
- R. Hekmatshoar, S. Majedi and K. Bakhtiari, Catal. Commun., 2008, 9, 307 CrossRef CAS PubMed.
- Y. M. Ren and C. Cai, Catal. Commun., 2008, 9, 1017 CrossRef CAS PubMed.
- J. M. Khurana and S. Kumar, Tetrahedron Lett., 2009, 50, 4125 CrossRef CAS PubMed.
- G. Shabitha, K. Harundhathi, K. Sudhakar, B. S. Sartry and J. S. Yadav, Synth. Commun., 2009, 39, 433 CrossRef.
- W. O. Sun, P. Zhang, J. Fan, S. H. Chen and Z. H. Zhang, Synth. Commun., 2010, 40, 587 CrossRef CAS.
- M. M. Khodaei, K. Bahrami and A. Farrokhi, Synth. Commun., 2010, 40, 1492 CrossRef CAS.
- D. Fang, H. B. Zhang and Z. L. Liu, J. Heterocycl. Chem., 2010, 47, 63 CAS.
- Y. Li, H. Chen, C. Shi and S. Ji, J. Comb. Chem., 2010, 12, 231 CrossRef CAS PubMed.
- S. Zavar, Arabian J. Chem., 2012 DOI:10.1016/j.arabjc.2012.07.011.
- S. Banerjee, A. Horn, H. Khatri and Gr. Sereda, Tetrahedron Lett., 2011, 52, 1878 CrossRef CAS PubMed.
- S. Nemouchi, R. Boulcina, B. Carboni and A. M. Debache, C. R. Chim., 2012, 15, 394 CrossRef CAS PubMed.
- J. Zheng and Y. Li, Mendeleev Commun., 2011, 21, 280 CrossRef CAS PubMed.
- S. S. Katkar, M. K. Lande, B. R. Arbad and S. T. Gaikwad, Chin. J. Chem., 2011, 29, 199 CrossRef CAS.
- J. Zheng and Y. Q. Li, Scholar Research Library, 2011, 3, 381 CAS.
- P. P. Salvi, A. M. Mandhare, A. S. Sartape, D. K. Pawar, S. H. Han and S. S. Kolekar, C. R. Chim., 2011, 14, 878 CrossRef CAS PubMed.
- M. Seifi and H. Sheibani, Catal. Lett., 2008, 126, 275 CrossRef CAS.
- H. Sheibani, M. Seifi and A. Bazgir, Synth. Commun., 2009, 39, 1055 CrossRef CAS.
- D. Kumar, V. B. Reddy, B. G. Mishra, R. K. Rana, M. N. Nadagouda and R. S. Varma, Tetrahedron, 2007, 63, 3093 CrossRef CAS PubMed.
- M. M. Heravi, B. A. Jani, F. Derikvand, F. F. Bamoharram and H. A. Oskooie, Catal. Commun., 2008, 10, 272 CrossRef CAS PubMed.
- G. M. Ziarani, A. Badiei, M. Azizi and P. Zarabadi, Iran. J. Chem. Chem. Eng., 2011, 30, 59 Search PubMed.
- K. Singh, J. Singh and H. Singh, Tetrahedron, 1996, 52, 14273 CrossRef CAS.
- X. S. Wang, D. Q. Shi, S. T. Tu and C. S. Yao, Synth. Commun., 2003, 33, 119 CrossRef CAS PubMed.
- S. J. Tu, Y. Gao, C. Guo, D. Shi and Z. Lu, Synth. Commun., 2002, 32, 2137 CrossRef CAS PubMed.
- J. T. Li, W. Z. Xu, L. C. Yang and T. S. Li, Synth. Commun., 2004, 34, 4565 CrossRef CAS PubMed.
- G. Kaupp, M. R. Naimi-Jamal and J. Schmeyers, Tetrahedron, 2003, 59, 3753 CrossRef CAS.
- (a) X. Fan, X. Hu, X. Zhang and J. Wang, Aust. J. Chem., 2004, 57, 1067 CrossRef CAS; (b) T. S. Jin, A. Q. Wang, X. Wang, J. S. Zhang and T. S. Li, Synlett, 2004, 5, 871 CrossRef PubMed.
- I. Devi and P. J. Bhuyan, Tetrahedron Lett., 2004, 45, 8625 CrossRef CAS PubMed.
- Y. Chen and H. Gu, Mater. Lett., 2012, 67, 49 CrossRef CAS PubMed.
- L. Kong, X. Lu and W. Zhang, J. Solid State Chem., 2008, 181, 628 CrossRef CAS PubMed.
- D. M. Pore, K. A. Undale, B. B. Dongare and U. V. Desai, Catal. Lett., 2009, 132, 104 CrossRef CAS PubMed.
- Z. K. Tang, L. Zhang and N. Wang, Science, 2001, 292, 3462 CrossRef PubMed.
- C. A. Grimes, E. C. Dickey, C. Mungle, K. G. Ong and D. Qian, J. Appl. Phys., 2001, 90, 4134 CrossRef CAS PubMed.
- H. Lin, H. Zhu, H. Guo and L. Yu, Mater. Lett., 2007, 61, 3547 CrossRef CAS PubMed.
- S. Rathod, B. Arbad and M. Lande, Chin. J. Catal., 2010, 31, 631 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra46598e |
| This journal is © The Royal Society of Chemistry 2014 |