A new type of anion receptor: pyrrolyl quinones†
Melissa Tapia-Juáreza,
J. Betzabe González-Camposa,
Claudia Contreras-Celedóna,
David Coronab,
Erick Cuevas-Yañezb and
Luis Chacón-García*a
aLaboratorio de Diseño Molecular, Instituto de Investigaciones Químico Biológicas, Universidad Michoacana de San Nicolás de Hidalgo, Morelia, Mich. 58066, México. E-mail: lchacon@umich.mx
bCentro Conjunto de Investigación en Química Sustentable UAEM-UNAM, Carretera Toluca-Atlacomulco Km. 14.5, Toluca, Estado de México 52000, México
First published on 18th December 2013
Abstract
The synthesis of a novel 2-dipyrrolyl-2,5-dimethyl-p-benzoquinone is described, and its interactions with fluoride were characterized. An unprecedented interaction between fluoride anions and the quinone ring was observed.
Over the last few decades, the syntheses and uses of anion receptors have constituted an area of interest in the chemical, biological, materials, and multidisciplinary sciences.1 The nonbonding Lewis base electrons on a crown ether, selective cation receptors,2 interact with cations, which act as Lewis acids. Anion receptors, such as calixpyrroles,3 interact with anions by forming hydrogen bonds. A large number of selective receptors, including multianion, cation, and anion receptors, have been identified.4
The search for novel synthetic compounds that can act as ion receptors has revealed an interesting novel interaction, the anion-π interaction, which has attracted attention in the area of supramolecular chemistry.5 Although this interaction is not entirely new,5,6 the applicability of the π-anion interactions was not recognized until the recent discovery of reversible anion–arene interactions.7 Anion-π interactions are potentially useful for the design of selective anion receptors, hosts, scaffolds, colorimetric sensors, or catalysts. The interactions may be involved in biological functions,8 particularly functions that involve proteins–anion interactions.9
π complexes, which were initially described as occurring in the solid state, involve the transfer of electron density between electron-deficient and electron-enriched systems.10 Anion-π interactions, which are defined as favorable noncovalent contacts between an electron-deficient aromatic system and an anion, comprise a certain class of π complexes.11
Electron-withdrawing groups bound to pyrrolyl compounds tend to increase the compound's binding affinity toward anions, such as fluoride.12 Quinones have carbonyl groups which could potentially interact with cations by Lewis acid–base interactions. Futhermore, some quinones have been examined as possible ion receptors,13–15 for example, benzoquinone has been reported to recognize an ion pair16,17 and chloranil to interact with calixpyrroles by non-covalent electron charge transfer.18 Nevertheless, anion–quinone interactions have been studied theoretically, so these compounds have been poorly examined as anion receptors even though they play important roles in biological systems. Also, although pyrrole compounds are a widely studied class of anion receptors, to our knowledge, no pyrrolyl–quinone compounds have been described to selectively recognize ions. We recently described the synthesis of the novel 2-(1,5-dimethyl-4-oxo-hexyl)-3-hydroxy-5-methyl-6-pyrrolyl-1,4-benzoquinone 1, which was designed to function as a ditopic receptor that could associate with ion pairs. The compound included an anion-recognizing region (a pyrrole hydrogen bond donor group) and a cation-recognizing region that engaged in Lewis acid interactions via a carbonyl side chain. This compound displayed good specificity toward fluoride binding.19 These results led us to design receptor 2 in an attempt to probe the efficacy of the anion-π interactions. We focused on a two-point recognition motif and utilized both a hydrogen bonding dipyrrolyl group and an electron-deficient redox-active quinone-based chromophore. Note that p-quinone is widely used as a transducing unit and that dipyrromethanes are efficient anion receptors via hydrogen bonds (Scheme 1).20
The synthesis of receptor 2 was carried out using a method described previously.19 2,5-Dimethyl-1,4-benzoquinone was reacted with meso-dimethyl-dipyrromethane to give 2 in a 42% yield (Scheme 2). Compound 2 was fully characterized by 1H and 13C NMR and by HRMS.‡ The 1H NMR spectrum of 2 revealed that the NH signal of the pyrrole (H-8) group was shifted to 10.5 ppm, 2.5–3.5 ppm downfield from the typical dipyrrole NH shift of 7–8 ppm. This suggested that the pyrrole formed a H-bond with the quinone carbonyl. The NOESY results confirmed the proximity of the pyrrole H-β to the methyl quinone. These results are interesting because the NH groups behave differently in the presence of an anion. This interaction is expected to lead to a more electron-deficient quinone, which was helpful in the present study.
The anion–guest properties of 2 were evaluated in the presence of fluoride or chloride tetrabutylammonium salts using 1H NMR titration techniques in deuterated acetonitrile at 298 K (see ESI†). The addition of fluoride first produced a change in the solution colour. The C–H (H-6) and CH3 quinone and pyrrole (H-11) signals disappeared upon the addition of 0.1 molar equivalents of the TBA salt, suggesting the presence of anion-π interactions between fluoride and the most electron-deficient quinone, which then perturbed the adjacent pyrrole group. As the salt was added, the NH (H-8) and NH (H-14) protons shifted downfield to 10.8 (Δδ 1 ppm) or 10.2 ppm (Δδ 1.8 ppm), respectively in an excess of FTBA (Fig. 1). This spectroscopic behavior was analogous to that observed in strong electronic interactions between lone-pair electrons of F-ion and π*-orbitals. The supramolecular interaction (anion-π) involved fluoride ions and π-electron deficient receptors.21
 | ||
| Fig. 1 1H NMR (in acetonitrile-d3 anhydrous at 298 K) of (a) compound 2 and (b) compound 2 upon addition of tetrabutylammonium fluoride. | ||
The addition of D2O to the NMR sample resulted in the reappearance of the signals, and deuterium exchange was observed at the NH pyrrole (Fig. 2a). Interestingly, no signals disappeared from the NMR spectra in the presence of chloride tetrabutylammonium. The 13C NMR spectra revealed the disappearance of signals attributed to a quinone ring (Fig. 2b). Changes in the NMR spectra were observed for compounds that interacted with anions via anion-π interactions.22,23
 | ||
| Fig. 2 (a) 1H NMR spectra of compound 2 with 0 and 0.08 fluoride equivalents and at the top, after addition of D2O. (b) 13C NMR spectra of compound 2 with 0 and 0.08 fluoride equivalents. | ||
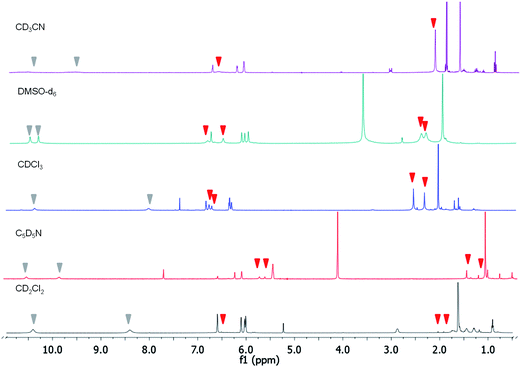
Titrations in chloroform-d, pyridine-d5, or dimethyl sulfoxide-d6 produced the same effects (Fig. 3). Having in mind that water could play a role in the interpretation of the results since it could hydrate fluoride, we did a titration of compound 2 in the presence of FTBA with an excess of water. There were not NH shifts and no broadening of the quinone signals were present. Also, no appreciable colour change was observed.
On the other hand, the 19F NMR spectrum revealed two signals that appeared upon the addition of the TBA salt. A first signal appeared at −151 ppm that, according to the 1H and 13C NMR spectra, suggested the presence of an interaction between the fluoride and the quinone. The second signal appeared at 109 ppm (Fig. 4). The downfield and upfield shifts in these signals were expected in view of anisotropy and inductive effects.
 | ||
| Fig. 4 19F NMR (470 MHz) titration plot of compound 2 upon addition of tetrabutylammonium fluoride in acetonitrile-d3 anhydrous at 298 K. | ||
The UV-Vis spectra,§ collected during a fluoride titration in CH3CN, are shown in Fig. 5. The formation of the complex was supported by the appearance of a broad band at approximately 434 nm in the UV-Vis spectrum. This feature corresponded to colour changes from red to purple to pale orange. Neither displacement of UV bands or colour change was observed in the presence of other anions (Fig. 6).
 | ||
| Fig. 5 UV-Vis titration of compound 2 in acetonitrile (5 × 10−4 M) solution. Upon addition of fluoride as TBA salt. (a) 0 to 30 equiv. (b) 0–3 equiv. (c) 4–14 equiv. and (d) 15–30 equiv. | ||
During sequential addition of up to 30 equiv. of FTBA to an acetonitrile solution of compound 2, three apparent isosbestic points were observed in UV-Vis spectra (Fig. 5). This result is in agreement with the data obtained by 1H NMR titrations, which suggest that three different complexes are successively formed upon FTBA addition (Scheme 3).
A comparative titration of meso-dimethyl-dipyrromethane, 1,5-dimethyl-1,4-dibenzoquinone and compound 2 was carried out with FTBA (see ESI†). Dipyrromethane did not show change in the colour of the solution upon to the addition of FTBA. On the other hand, benzoquinone experimented a change from yellow to increasing the absorbance in the UV spectra, thus showing an interaction with F−. Moreover, neither dipyrromethane nor benzoquinone were hypsochromically shifted upon the addition of up to 20 eq. of FTBA. The spectroscopic behavior of 1 and 2 was different. In the NMR titrations toward FTBA, compound 1 showed only NH shifts upon the addition of the fluoride salt. Consequently, whereas compound 1 showed only one isosbestic point in the UV-vis spectra compound 2 showed apparently three (see ESI†).
Conclusions
The anion-π interaction energy has been reported to be comparable to the energy of a hydrogen bond (20–50 kJ mol−1). Our results revealed that the first interaction that formed involved the quinone group, and the second involved the NH groups, indicating that the affinity of the quinone for fluoride ions was higher than the corresponding affinity of the NH groups. Anion–quinone interactions have been studied theoretically;24 however, to our knowledge, this is the first experimental evidence supporting the existence of this interaction. Compound 2 provides a novel ditopic ligand with properties that differ from those of quinone or pyrrole, which act as anion receptors, predominantly via an anion-π interaction.Notes and references
- B. L. Schottel, H. T. Chifotides and K. R. Dunbar, Chem. Soc. Rev., 2008, 37, 68–83 RSC.
- C. J. Pedersen, J. Am. Chem. Soc., 1967, 89, 7017–7036 CrossRef CAS.
- P. A. Gale, J. L. Sessler and V. Kral, Chem. Commun., 1998, 1–8 RSC.
- S. K. Kim and J. L. Sessler, Chem. Soc. Rev., 2010, 39, 3784–3809 RSC.
- C. L. Jackson and W. F. Boos, Am. Chem. J., 1898, 20, 444–455 CAS.
- C. L. Jackson and F. H. Gazzolo, Am. Chem. J., 1900, 23, 376–396 CAS.
- O. B. Berryman and D. W. Johnson, Chem. Commun., 2009, 3143–3153 RSC.
- H. T. Chifotides and K. R. Dunbar, Acc. Chem. Res., 2013, 46, 894–906 CrossRef CAS PubMed.
- A. Robertazzi, F. Krull, E.-W. Knappb and P. Gamez, CrystEngComm, 2011, 13, 3293 RSC.
- R. S. Mulliken, J. Am. Chem. Soc., 1952, 74, 811–824 CrossRef CAS.
- D. Quiñonero, C. Garau, C. Rotger, A. Frontera, P. Ballester, A. Costa and P. M. Deyà, Angew. Chem., Int. Ed., 2002, 41, 3389 CrossRef.
- T. Ghosh, B. G. Maiya and M. W. Wong, J. Phys. Chem. A, 2004, 108, 11249–11259 CrossRef CAS.
- N. Kerdpaiboon, B. Tomapatanaget, O. Chailapakul and T. Tuntulani, J. Org. Chem., 2005, 70, 4797–4804 CrossRef CAS PubMed.
- H. Kim, Bull. Korean Chem. Soc., 2010, 31, 3115–3117 CrossRef.
- B. Wannalerse, T. Tuntulani and B. Tomapatanaget, Tetrahedron, 2008, 64, 10619–10624 CrossRef CAS PubMed.
- M. D. Lankshear, A. R. Cowley and P. D. Beer, Chem. Commun., 2006, 612–614 RSC.
- A. J. McConnell, C. J. Serpell and P. D. Beer, New J. Chem., 2012, 36, 102–112 RSC.
- S. Shao, Y. Guo, L. He, S. Jiang and X. Yu, Tetrahedron Lett., 2003, 44, 2175–2178 CrossRef CAS.
- L. Chacón-García, M. Valle-Sánchez and C. Contreras-Celedón, Lett. Org. Chem., 2013, 10, 632–636 CrossRef PubMed.
- J. L. Sessler, S. Camiolo and P. A. Gale, Coord. Chem. Rev., 2003, 240, 17–55 CrossRef CAS.
- S. Guha and S. Saha, J. Am. Chem. Soc., 2010, 132, 17674–17677 CrossRef CAS PubMed.
- M. Khandelwal, I.-Ch. Hwang and P. C. R. Nair, J. Fluorine Chem., 2012, 135, 339–343 CrossRef CAS PubMed.
- Z. Xu, N. J. Singh, S. K. Kim, D. R. Spring, K. S. Kim and J. Yoon, Chem.–Eur. J., 2011, 17, 1163–1170 CrossRef CAS PubMed.
- A. Ebrahimi, M. Habibi and O. Sayyadi, J. Mol. Struct.: THEOCHEM, 2008, 859, 46–50 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Spectroscopic information of compound 2, 1H NMR and UV-vis titrations of compound 2 and UV-vis titration of compound 1, meso-dimethyl-dipyrromethane, 1,5-dimethyl-1,4-dibenzoquinone with FTBA. See DOI: 10.1039/c3ra46594b |
‡ To a solution of dipyrromethane (0.129 g, 0.74 mmol) dissolved in CH2Cl2 (2 ml) was added 2,5-dimethyl1-1,4-benzoquinone (0.1 g, 0.73 mmol), then SiO2 (1.3 g) was added and the solvent was removed. The reaction was kept at room temperature for 17 h. The residue was purified by flash column chromatography (Hex![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc 9 EtOAc 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give compound 2 as a purple solid; yield 0.096 g, 42.47%, mp 115–118 °C. 1H-NMR (400 MHz, CDCl3): δ 10.36 (s, 1H), 7.91 (s,1H), 6.72–6.67 (m, 1H), 6.63 (dd, J = 3.8, 2.7 Hz, 1H), 6.57 (dd, J = 3.0, 1.5 Hz, 1H), 6.22–6.17 (m, 2H), 6.15 (td, J = 3.0, 1.6 Hz, 1H), 2.24 (d, J = 3.6 Hz, 3H), 2.01 (d, J = 1.5 Hz, 3H), 1.72 (s, 6H). 13C-NMR (101 MHz, CDCl3): δ 190.9, 186.9, 144.5, 144.3, 138.0, 133.8, 133.6, 131.5, 124.6, 118.03, 117.09, 108.1, 106.2, 104.0, 35.6, 28.9, 15.9, 14.7. HRMS M + 1, calc. 309.152478, obs. 309.1378. 1) to give compound 2 as a purple solid; yield 0.096 g, 42.47%, mp 115–118 °C. 1H-NMR (400 MHz, CDCl3): δ 10.36 (s, 1H), 7.91 (s,1H), 6.72–6.67 (m, 1H), 6.63 (dd, J = 3.8, 2.7 Hz, 1H), 6.57 (dd, J = 3.0, 1.5 Hz, 1H), 6.22–6.17 (m, 2H), 6.15 (td, J = 3.0, 1.6 Hz, 1H), 2.24 (d, J = 3.6 Hz, 3H), 2.01 (d, J = 1.5 Hz, 3H), 1.72 (s, 6H). 13C-NMR (101 MHz, CDCl3): δ 190.9, 186.9, 144.5, 144.3, 138.0, 133.8, 133.6, 131.5, 124.6, 118.03, 117.09, 108.1, 106.2, 104.0, 35.6, 28.9, 15.9, 14.7. HRMS M + 1, calc. 309.152478, obs. 309.1378. |
| § Compound 2 was re-crystallized prior to measurements. Solvents purchased for UV-vis measurements were grade HPLC. |
| This journal is © The Royal Society of Chemistry 2014 |