Hepatotoxicity induced by ZnO quantum dots in mice
Abstract
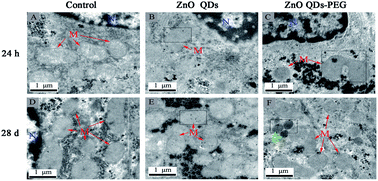
ZnO quantum dots (QDs) with unique optical properties are potential useful tools for biological labeling and biosensing. With the increasing use of ZnO QDs, the toxicity evaluation of ZnO QDs is urgent. In this study, the hepatotoxicity including serum aminotransferases (ALT and AST), antioxidant enzymes (CAT, GSH-Px and SOD), lipid peroxidation and ultrastructure were evaluated after consecutive intravenous injection of ZnO QDs and ZnO QDs–PEG for 7 days in mice. Both ZnO QDs and ZnO QDs–PEG did not affect the coefficient of liver and the levels of serum aminotransferases. The antioxidant enzymes and lipid peroxidation had significant change after injecting 5 mg kg−1 ZnO QDs in 24 h, but all of these parameters returned to control levels in 28 days. ZnO QDs–PEG had a less harmful effect on antioxidant enzymes and malondialdehyde than ZnO QDs at the same dose. According to the results of hepatocyte ultrastructure, both ZnO QDs and ZnO QDs–PEG were located in the mitochondrion and induced nuclear malformation in 24 h. The ultrastructure of hepatocyte was as normal as of the control group in 28 days and ZnO QDs were mainly trapped in the mitochondrion while ZnO QDs–PEG mainly accumulated in the lysosomes. These findings would be helpful for wide use of quantum dots based bioimaging and biomedical applications in the future.

 Please wait while we load your content...
Please wait while we load your content...