Effect of volatile solvent infiltration on optical and electrical characteristics of porous photonic structures†
Abstract
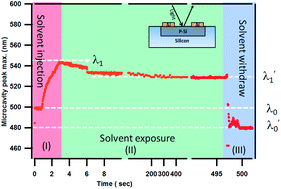
The infiltration of small chain alcohols into the deep nano sized pores of one dimensional porous silicon (PS) based photonic structures have been continuously monitored against time by simultaneous electrical and optical measurements. The in situ optical reflection studies during volatile solvent exposure reveal several dynamic processes; within a limited time duration of solvent exposure the microcavity resonant peak shifts towards higher wavelength, and after prolonged exposure and drying the cavity resonant peak shifts to a new semi-permanent lower wavelength. In situ optical and electrical responses from PS photonic structure-based low-cost multifunctional devices reveal their potential application for a wide range of chemical and biological species detection and monitor their sensor dynamic processes.

 Please wait while we load your content...
Please wait while we load your content...