Polymer-based acoustic streaming for improving mixing and reaction times in microfluidic applications
Abstract
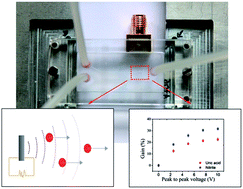
This paper reports on a polymer-based piezoelectric transducer used in microfluidic structures for generating acoustic waves, and consequently, the acoustic streaming phenomenon, that will improve the mixing of fluids. The piezoelectric transducer is based on a poly(vinylidene fluoride-trifluoroethylene), P(VDF-TrFE), copolymer with conductive transparent electrodes of aluminum doped zinc oxide (AZO). The efficiency of the optimized piezoelectric P(VDF-TrFE) transducer on mixing fluids was studied using two diagnostic kits for the quantification of uric acid and nitrite in blood. In both cases, acoustic streaming reduced the reaction time for the quantification of each biomolecule when compared to the reaction time achieved only by diffusion, with gains of 23% and of 32% for the uric acid and nitrite, respectively. These results contribute to increase the efficiency in fluid mixing and therefore to improve the overall performance of miniaturized analysis systems.

 Please wait while we load your content...
Please wait while we load your content...