Gas-sensing properties of p-type α-Fe2O3 polyhedral particles synthesized via a modified polyol method
Abstract
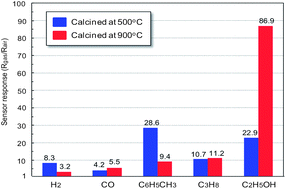
The gas sensing properties of polyhedral α-Fe2O3 particles were investigated. The polyhedral α-Fe2O3 particles were synthesized via a modified polyol method with the addition of an extra amount of NaBH4 in ethylene glycol, and fabricated to a thick gas sensing film after calcination at 500 °C and 900 °C. The polyhedral α-Fe2O3 particles exhibited a p-type nature, which is a new result opposite to other results of recent reports of semiconductor gas sensors using α-Fe2O3. In particular, we suggest that the difference between structure and morphology of α-Fe2O3 particles can lead to p-type and n-type characterization. In addition, we suggested that Na ions from NaBH4 were incorporated into α-Fe2O3 oxide. They are the cause of the generation of holes in α-Fe2O3 oxide that led to the p-type nature of α-Fe2O3 oxide. The measurement of the sensor response to hydrogen, carbon monoxide, toluene, propane and ethanol revealed that the sensor device synthesized using polyhedral α-Fe2O3 particles is sensitive to hydrocarbons and ethanol. Importantly, the sensor device using polyhedral α-Fe2O3 particles calcined at 900 °C was strongly sensitive to ethanol due to the porous structure of the α-Fe2O3 particles.

 Please wait while we load your content...
Please wait while we load your content...