A novel process for high efficiency recovery of rare earth metals from waste phosphors using a sodium peroxide system†
Abstract
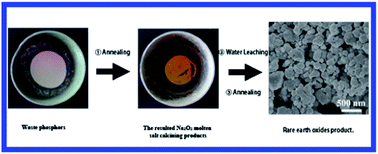
A novel process for recovering Rare-Earth Elements (REEs) from waste phosphors has been developed. The treatment of waste phosphors by a Na2O2 molten salt calcining process is investigated. The results demonstrate that the calcination temperature and Na2O2-to-waste mass ratio have significant influences on the REEs extraction. Meanwhile, Ce and Tb of waste phosphors are converted to Na2CeO3 and Na2TbO3 during the molten salt calcining process, respectively. The optimal conditions in terms of temperature, time and Na2O2-to-waste mass ratio are 650 °C, 50 min and 1.5 : 1, respectively, under which more than 99.9% REEs are recovered. The shrinking core model with mass diffusion in the product layer controlled process has been found most applicable for the decomposition of waste, with the apparent activation energy of 76.7 kJ mol−1. This novel process holds promising potentials for low-cost and high efficiency of recycling waste phosphors on a large industrial scale.

 Please wait while we load your content...
Please wait while we load your content...