Design of ferromagnetism in Co-doped SnO2 nanosheets: a first-principles study
Hang-xing Luan,
Chang-wen Zhang*,
Feng Li,
Ping Li,
Miao-juan Ren,
Min Yuan,
Wei-xiao Ji and
Pei-ji Wang
School of Physics and Technology, University of Jinan, Jinan, Shandong 250022, People's Republic of China. E-mail: zhchwsd@163.com; Fax: +86-531-82765976; Tel: +86-531-82765976
First published on 27th January 2014
Abstract
First-principles calculations are performed to study the structural, electronic and magnetic properties of Co-doped SnO2 nanosheets (NSs), using the generalized gradient approximation (GGA) plus Hubbard U method. We find that two Co atoms have a clustering tendency and the magnetic interactions between them exhibit a ferromagnetic (FM) coupling, while the appearance of an oxygen vacancy (VO) turns it into an antiferromagnetic (AFM) order. When the Li atom is codoped into Co-doped SnO2NS, the interactions between Co atoms are rehabilitated to FM coupling with a high Curie temperature (TC) of 850 K. The electronic structure analysis reveals that this is mainly attributed to the hole-induced double-exchange mechanism from s–d hybridizations between Li and Co, which finally activates a long-range FM coupling between two Co atoms. These findings could be very useful in nano-material design for spintronics.
I. Introduction
Dilute magnetic semiconductors (DMSs) have attracted extensive attention in the past few years due to their potential applications in spintronic devices.1,2 Since the observation of high temperature ferromagnetism (TC close to 650 K) with a giant magnetic moment of 7.5 ± 0.5 μB/Co by Ogale et al.,2 the transition metal (TM) doped SnO2 DMSs have been identified as some of the most promising candidates. However, the magnetic origin of TM-doped SnO2 is quite controversial nowadays, because the magnetic behavior of TM-doped SnO2 is strongly dependent on the preparation methods and is poorly reproducible.3,4 For example, ferromagnetism with high TC was observed in Fe-,5 Cr-,6 V-,7 and Ni-doped8 SnO2, and especially in the case of Mn-doped it shows a very large magnetic moment up to 20.0 μB at low doping concentration, which is far above the spin moment of Mn ion.9 To clarify the FM nature of TM-doped cases theoretically, many calculations based on density-functional theory (DFT) have been carried out, which recognized that the VO may be responsible for the promotion of ferromagnetism. Those are partially in consistent with experimental results.5,8 However, in comparison to a considerable amount of experimental data, the origin of FM order in TM-doped SnO2 is still not well understood.Recently, focus has shifted to the low-dimensional SnO2NSs, of which the formation mechanism and relatively stability have been discussed.10–15 The synthesis of the SnO2NSs opens up new possibilities for fabricating SnO2 based spintronics devices. Theoretically, Rahman et al.16 reported the spin-polarized states induced by C substitutions in SnO2 surface, whereas no magnetism is observed if C atom is doped into inner oxygen site in SnO2 thin film. These demonstrate that the surface quantum confinement effects play an important role for ferromagnetism in SnO2 nanostructure. However, how to theoretically design and manipulate the room-temperature ferromagnetism in SnO2NS has seldom been reported up to date.
Based on our theoretical results and orbital hybridization model, the origin of the long-range ferromagnetism in (Co, Li)-codoped systems has been discovered. The two Co atoms have a clustering tendency and the magnetic interactions between them exhibit a FM coupling, while the appearance of VO turns it to be an AFM order. When the Li atom is codoped into SnO2NS, the interactions between Co atoms are rehabilitated to FM coupling with a high Curie temperature (TC) of 850 K. These findings and their fundamental mechanism should be important in the successful fabrication of the excellent SnO2NS for spintronics applications.
II. Computational details
We preformed first-principles calculations using the Vienna ab initio simulation package (VASP) code17 based on density-functional theory (DFT) with the projector augmented wave (PAW) method18 in conjunction with the generalized gradient approximation (GGA) and strong correlation effects were introduced by means of GGA + U method,19 where U (U = 3.0 eV) has been applied to Co 3d orbital since further correction for the host material has little impact on the magnetic order.20 The electronic wave functions were expanded by plane waves with the cutoff energy of 400 eV. Two-dimensional periodic boundary conditions were applied to the Co-doped SnO2NS while a vacuum spacing of 20 Å is set along the direction perpendicular to the SnO2NS surface to avoid the mirror interaction. All atomic positions in all structures were optimized until the forces had converged to be less than 0.01 eV Å−1. For k-space integration, we use a grid of 5 × 5 × 1 k points in the first Brillouin zone.III. Results and discussions
Firstly, we study the geometric structure of perfect SnO2NS, which is modeled with a 4 × 4 SnO2 supercell in the x-y plane containing sixteen SnO2 units, with a vacuum region of 20 Å perpendicular to the SnO2 surface, as shown in Fig. 1(a). Each Sn atom is in the center of a pseudo-octahedron composed of six-oxygen ring, in which four O atoms locate in the same plane with central Sn atom, while the otherwise two O atoms occupy the vertex sites. After structural optimization, both Sn and O sublattice are equilateral triangle with the lattice constant of 3.20 Å, less than the experimental lattice constant of bulk SnO2 (4.74 Å).21 This can be attributed to a symmetric shrinking, being similar to TiO2 nanosheets.22 The averaged Sn–O bond length (2.11 Å) is also known. Different from the planar structure in graphene,23 the SnO2NS forms a sandwiched structure, where Sn atoms are located in the middle of two-layered oxygen atoms, as shown of side view in Fig. 1(b).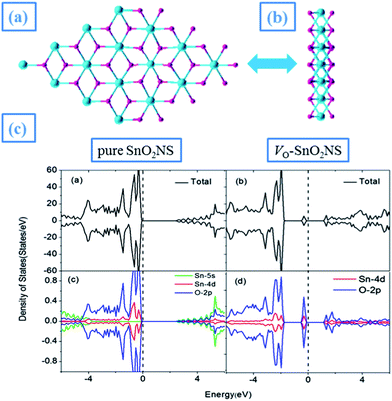 | ||
| Fig. 1 (a) Top view, (b) side view of pure SnO2NS; (c) DOS of pure and VO-doped SnO2NS. Turquoise and pink balls represent Sn and O atoms, respectively. | ||
In Fig. 1(c), we present the total and partial density of states (DOS) for perfect SnO2NS. It can be seen that the valence band maximum (VBM) near the Fermi level (EF) mainly consists of Sn 4d and O 2p states, while the conduction band minimum (CBM) comes from Sn 5s state. Thus the VBM–CBM gap is about 2.75 eV, larger than the theoretical value of bulk SnO2 (about 1.90 eV) that we calculated by GGA. This can be attributed to nanometer size effect. In addition, the spin-polarized calculations indicate that the electron wave function in spin-up and spin-down channels is symmetric, exhibiting a nonmagnetic behavior. This is because that Bader analysis shows that the Sn atom is in a +2 state due to the donation of its two valence electrons to neighboring oxygen atoms, which are in a −1 state. As a result, no unpaired electrons exist in perfect SnO2NS, in good agreement with previous results.24
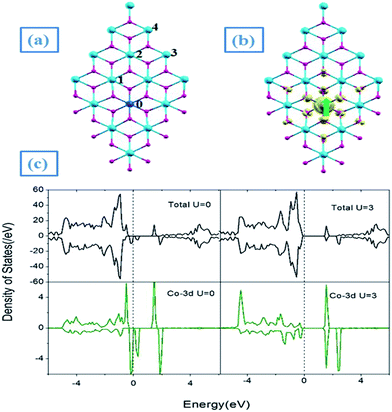
Next, we begin to study the effect of Co substitution for Sn atoms in SnO2NS. We placed a single Co atom in SnO2NS by removing one Sn atom, corresponding to a defect concentration of 2.08%, as shown in Fig. 2(a). After structural relaxation, the O–Co–O layered sandwich configurations are preserved, where the Co dopant is located in the middle of two-layered oxygen atoms. The Co–O bond length contracts by 0.17 Å significantly due to smaller ionic radius of Co atom, while the Sn–O bond length (2.11 Å) changes less. It is of note that the Co substitution for Sn atom results in the spin-polarized states due to the asymmetrical distribution of the wave functions of spin-up and spin-down channels on Co-3d states near the EF [Fig. 2(c)]. To further verify the localization of the magnetic moment, we plot the net magnetization m(r) = ρ↑(r) − ρ↓(r), where ρ↑(↓)(r) is the total charge density in the ↑↓polarized channel. The m(r) is plotted for a single isolated Co atom as shown in Fig. 2(b). The spin-density plots show clearly a strong localized magnetic moment at the Co site, which has a strong “up” d character. Meanwhile, in the direction of the bond with Co atom, the nearest-neighboring O atoms has slightly “up” p like character due to hybridization of Co-3d and O-2p states. The total magnetic moment is found to be 1.0 μB, in which the local magnetic moment is around 0.86 μB for Co atom. These can be understood as follows: a Co impurity has five quasi-localized electrons on Co-3d states. Under the symmetry notations of the local octahedral crystal field, the Co-3d states are split into the triply-degenerate t2g and double-degenerate eg state, the latter occurring at the higher energy than the former. Due to spin polarization, the occupied electronic configuration of Co4+ is d5[(t↑2g)3(t↓2g)2], exhibiting the half-metallic behavior with 100% spin-polarized current at the EF [Fig. 2(c)]. Due to the poor description of strong Coulomb correlation and exchange interaction between electrons in GGA, we check these results with GGA + U (U = 3 eV).25 It is of note that the partial occupied Co-t2g spin-down states are further split into the double-degenerate e2 and single a1 states. So, the spin-down electrons of Co4+ ion becomes d2[(e↓2)2(a↓1)0], clearly opening the gap between the doubly-degenerate e2 and singly-degenerate a1 states in the vicinity of Fermi level. As s result, the Co-doped SnO2NS transforms from half-metal to semiconductor when U is included on Co-3d states, as shown in Fig. 2(c).
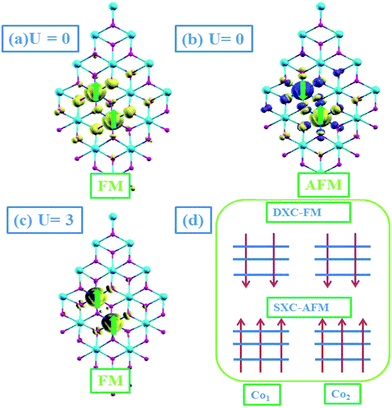
To analyze its magnetic nature, we studied the coupling between two Co atoms in SnO2NS. The Co dopants are still substituted for Sn sites, corresponding to the doping concentration of 4.17%. Since there are only four nonequivalent sites for the incorporation of Co dopants into SnO2NS, we perform spin-polarized calculations for these configurations, such as I(0,1), II(0,2), III(0,3), and IV(0,4), as shown in Fig. 2(a). For each configuration, the magnetic energy difference, ΔEFM = EAFM − EFM, and the relative energy Δε with respect to the ground state of configurations I(0,1) are also presented in Table 1. It is of note that I(0,1) is energetically the lowest among four structures, demonstrating that it is the ground state and Co dopants have a clustering tendency, whether the U is considered or not. The ΔEFM in the ground state I(0,1) is found to be 14 meV, lower than that of AFM state. However, this difference decreases to be 2 meV when U is applied to Co-3d orbital, indicating the AFM and FM state might coexist. Especially, AFM states can be observed in the structure III(0,3) and IV(0,4) when the distance between two Co atoms is larger than 6.0 Å. The magnetic interactions could be understood as the competition between two exchange interactions: the super-exchange (SXC) interaction between the filled t↓2g electrons which gives a result of AFM, and the double-exchange (DXC) interaction between the partially filled t↑2g orbitals, leading to be FM.26,27 The latter one depends on the electron hopping between the neighboring Co atoms, and the local atomic arrangements and local orbital ordering of the two t↓2g electrons of the neighboring atoms, which control the magnitude of the two competing interactions. If the orbital orientation is such that the hopping is small, the AFM-SEC dominates as in case III and IV. The reason of the formation of SXC-AFM is that the triple degenerate spin-up orbitals of two Co atoms (Co1,Co2) are fully occupied and the electrons of Co2 atom have an inversion (changes from t↑↑↑2g to t↓↓↓2g), resulting in an indirect hopping between two Co atoms by the bonding O atoms. When the electron hopping is relatively large, the FM-DXC overwhelms the AFM-SXC, resulting in a net FM interaction as in case I. The plot of Fig. 3(d) illustrates this competition.
| Configurations | dCo–Co (Å) | Δε (meV) | ΔE (meV) | Coupling | MT (μB) | MCo (μB) |
|---|---|---|---|---|---|---|
| U = 0 | ||||||
| (0,1) | 3.10 | 0 | 14 | FM | 2 | 0.88 |
| (0,2) | 5.50 | 87 | 208 | FM | 2 | 0.88 |
| (0,3) | 6.35 | 185 | 0 | PM | 0 | — |
| (0,4) | 8.45 | 140 | 0 | PM | 0 | — |
| U = 3 | ||||||
| (0,1) | 3.11 | 0 | 2 | PM | 2 | 1.2 |
| (0,2) | 5.51 | 61 | 0 | PM | 0 | — |
| (0,3) | 6.35 | 146 | −401 | AFM | 0 | −1.2/1.2 |
| (0,4) | 8.45 | 103 | −406 | AFM | 0 | −1.2/1.2 |
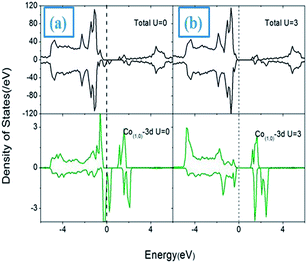
In Fig. 3(a)–(c), we also present the spin-density distributions of SnO2NS in the ground state I(0,1) with U = 0 and U = 3 eV, respectively. It can be seen that the net spin magnetic moment (0.88 μB) introduced by Co atom are localized in Co-3d orbitals [Fig. 3(a)], while the small contributions from the neighboring oxygen atoms (0.14 μB) are also obtained. When U = 3 eV is included, the strong coulomb interaction U increases clearly the localization of Co-3d electrons due to the further split of t2g states in spin-down channel [Fig. 3(c)], resulting in a net magnetic moment 0.95 μB. Fig. 4 presents the corresponding total and partial DOS with U = 0 and U = 3 eV, respectively. It is of note that, similar to the isolated substituted case, the transitions from half-metal to semiconductor properties are still observed.
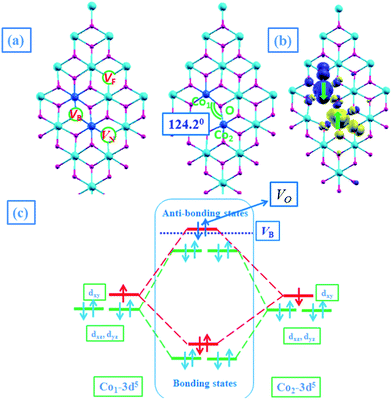
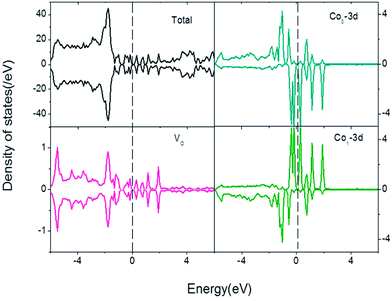
It is reported that VO is more likely to appear in the SnO2NS. In order to analyze the effect of VO on the magnetic coupling, we consider three types of VO sites on SnO2NS, termed as bridging-VO, neighboring-VO, and further-VO, and distinguish specific VO occupation sites by marking VB, VN, and VF, as shown in Fig. 5(a). In Table 2, we list the calculated total energies with respect to the VB occupation sites. The formation energy of this system could be defined as
| Ef = E(VO–Co–SnO2) + E(O) − E(Co–SnO2), |
| Locations (VO) | Δε (meV) | ΔE (meV) | Coupling | MT (μB) | MCo (μB) |
|---|---|---|---|---|---|
| VB | 0 | −109 | AFM | 0 | −1.6/1.6 |
| VN | 745 | 0 | PM | 0 | — |
| VF | 1242 | 0 | PM | 0 | — |
On the other hand, another possible explanation of the AFM mechanism on Co–VB–Co complex is that the coupling between Co atoms depends on the electron distributions in Co orbitals. The systematic plot of d level occupation is shown in Fig. 5(c). The presence of VO makes the point group lower from Oh to C4v. The t2g states are no longer degenerate, further splitting into one doubly degenerate e state (dxz, dyz) and one singly degenerate a state (dxy), while the eg state is splitting into two single degenerate a1(dz2) and a2(dx2−y2) states. We don't concern a1 and a2 states due to their high energy. The bonding levels of e and a states and the anti-bonding level of e state are fully filled by Co-3d electrons in spin-up and spin-down channels. When the SnO2NS is in an XY plane, due to the crystal-field splitting, O-2p levels are also split into one doubly degenerate state (px, py) and one singly degenerate state (pz), in which the latter occurs with the higher energy. When one O atom is removed, O-pz orbital will preferentially lose its two electrons, resulting in the anti-bonding level of singly degenerate a state fully occupied. Hence, the oxygen-deficient Co-doped SnO2NS is an AFM order. Thus, we conclude that the strength of AFM interactions between the Co atoms is determined by not only the AFM-SEC mechanism but also the crystal-field induced orbital energy splitting in Co-doped SnO2NS.
Recent experiments reported that Li can be easily incorporated into SnO2 lattice.29 Hence, it is expected that Li co-doping should introduce redundant positive charges into the lattice and neutralize the additional charges introduced by Co dopants, and thus can confine the formation of AFM order between Co atoms, thus promotes ferromagnetism in Co-doped SnO2NS. To check this scenario, we consider four configurations, i.e., (i) two Co atoms with an intermediate Li substituting for O atom (Co–LiO–Co complex) [1.08 eV], (ii) two Co atoms with a neighboring Li substituting for O atom [−2.00 eV], (iii) two Co atoms with a distant Li substituting for O atom [−0.82 eV], and (iv) two Co atoms with Li substitution for Sn sites (Co–LiSn–Co complex) [−2.40 eV]. We also define the formation energy as
| Ef = E(Li–Co–SnO2) + E(Sn,O) − E(Co–SnO2) − E(Li), |
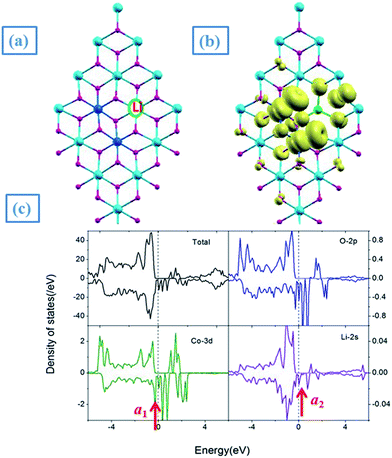 | ||
| Fig. 7 (a) The most stable structure, (b) spin density distribution and (c) DOS of (Co–Li)-codoped SnO2NS. | ||
To further clarify the nature of the effort of Li-codoping on the electronic and magnetic properties, we presented the DOS for the (Co, Li)-codoped SnO2NS, as shown in Fig. 7(c). As for Co mono-doped SnO2NS, the Co ion introduces a donor state within the band gap, as labeled as a1. In contrast, Li has one less valance electron than Sn, the substitution of Li on Sn site creates a impurity state near EF labeled as a2 in the gap. When Co and Li are co-doped into SnO2NS, we find that a charge transfer from a1 to a2 as illustrated by the arrows in Fig. 7(c). As a result, the a1 state of Co is partially filled, while the a2 state of Li is filled and finally pushed down EF. The results of charge transfer lead to not only the charges induced by Co and Li are mutually counteracted so that the formation of surface VO is effectively suppressed, but also the magnetization of Co ion is notably enhanced: the moment of Co is increased from 0.88 μB/Co for Co-doped SnO2 to 1.19 μB/Co for (Co, Li)-codoped SnO2NS.
On the other hand, the EF is significantly lower and the orbitals near EF also have a change due to the surface effect, making Co-3d states mix substantially with VBM of the host. Thus, the orbital change near EF favors a DEC interaction on the surface. In addition, the increase in the VB hybridization enhances the exchange coupling between Co atoms, and finally makes the FM interaction preferred over AFM one. When Li is codoped into this system, the VBs of this system are further shifted toward the lower energy, therefore the s bands of Li are favored to hybridize entirely with the Co 3d bands, as shown by the spin-density distributions in Fig. 7(b). This hybridization leads the holes on the acceptor levels passivated partially by donor levels, making EF shift towards impurity band, thus exhibiting half-metallic properties. These favor the carriers hopping between Co and the host atoms, and thus can help overcoming the difficulty in the enhancement of the low mobility of carriers in wide-band-gap DMSs. As a result, this magnetic exchange creates a significant enhancement on FM–DXC, and finally activates a long-range FM coupling between the Co atoms.
IV. Conclusions
In summary, by means of DFT calculations, we established a charge-passivated codoping approach to stabilize and enhance its ferromagnetism in Co-doped SnO2NS system as a prototype material for the DMS oxides. It is found the Co atoms have a clustering tendency and the magnetic interactions between them exhibit a FM state, while the appearance of VO turns it to an AFM coupling. When the Li atom is codoped into SnO2NS to substitute for Sn atom, the interactions between Co atoms are rehabilitated to FM coupling. The electronic structure analysis reveals that this is principally attributed to the hole-induced double-exchange interactions due to s-d hybridizations between Li and Co, which finally activates a long-range FM coupling between Co atoms. Our proposed model about the long-range ferromagnetism may be very useful to design the new spintronic materials.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant nos 61172028, 11274143 and 11304121), the Natural Science Foundation of Shandong Province (Grant no. ZR2010EL017), and Technological Development Program in Shandong Education Department (Grant no. J10LA16).References
- S. A. Wolf, D. D. Awschalom, R. A. Buhrman, J. M. Daughton, S. von Molnár, M. L. Roukes, A. Y. ChtchelkanVOa and D. M. Treger, Science, 2001, 294, 1488 CrossRef CAS PubMed.
- S. B. Ogale, R. J. Choudhary, J. P. Buban, S. E. Lofland, S. R. Shinde, S. N. Kale, V. N. Kulkarni, J. Higgins, C. Lanci, J. R. Simpson, N. D. Browning, S. Das Sarma, H. D. Drew, R. L. Greene and T. Venkatesan, Phys. Rev. Lett., 2003, 91, 077205 CrossRef CAS.
- D. Menzel, A. Awada, H. Dierke, J. Schoenes, F. Ludwig and M. Schilling, J. Appl. Phys., 2008, 103, 07D106 CrossRef PubMed.
- P. Wu, B. Zhou and W. Zhou Appl, Phys. Lett., 2012, 100, 182405 Search PubMed.
- J. M. D. Coey, A. P. Douvalis, C. B. Fitzgerald and M. Venkatesan, Appl. Phys. Lett., 2004, 84, 1332 CrossRef CAS PubMed.
- N. H. Hong, J. Sakai, W. Prellier and A. Hassini, J. Phys.: Condens. Matter, 2005, 17, 1697 CrossRef CAS.
- N. H. Hong and J. Sakai, Phys. B, 2005, 358, 265 CrossRef CAS PubMed.
- N. H. Hong, A. Ruyter, W. Prellier, J. Sakai and N. T. Huong, J. Phys.: Condens. Matter, 2005, 17, 6533 CrossRef CAS.
- C. B. Fitzgerald, M. Venkatesan, L. S. Dorneles, R. Gunning, P. Stamenov, J. M. D. Coey, Li. A. Stampe, R. J. Kennedy, E. C. Moreira and U. S. Sias, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 74, 115307 CrossRef.
- R. S. Yang and Z. L. Wang, J. Am. Chem. Soc., 2006, 128, 1466–1467 CrossRef CAS PubMed.
- C. Wang, Y. Zhou, M. Y. Ge, X. B. Xu, Z. P. Zhang and J. Z. Jiang, J. Am. Chem. Soc., 2010, 132, 46–47 CrossRef CAS PubMed.
- Q. Kuang, C. S. Lao, Z. L. Wang, Z. X. Xie and L. S. Zheng, J. Am. Chem. Soc., 2007, 129, 6070–6071 CrossRef CAS PubMed.
- C. Wang, M. Y. Ge and J. Z. Jiang, Appl. Phys. Lett., 2010, 97, 042510 CrossRef PubMed.
- C. Wang, G. H. Du, K. Ståhl, H. X. Huang, Y. J. Zhong and J. Z. Jiang, J. Phys. Chem. C, 2012, 116, 4000–4011 CAS.
- C. Wang, Q. Wu, H. L. Ge, T. Shang and J. Z. Jiang, Nanotechnology, 2012, 23, 075704 CrossRef PubMed.
- G. Rahman and V. M. García-Suárez, Appl. Phys. Lett., 2010, 96, 052508 CrossRef PubMed.
- G. Kresse and J. Furthmuller, Phys. Rev. B: Condens. Matter, 1996, 54, 11169 CrossRef CAS.
- G. Kresse and D. Joubert, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59(3), 1758 CrossRef CAS.
- J. P. Perdew and Y. Wang, Phys. Rev. B, 1986, 33, 8800 CrossRef.
- H. Weng, X. Yang, J. Dong, H. Mizuseki, M. Kawasaki and Y. Kawazoe, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 69, 125219 CrossRef.
- B. Cheng, J. M. Russell, W. S. Shi, L. Zhang and E. T. Samulski, J. Am. Chem. Soc., 2004, 126, 5972–5973 CrossRef CAS PubMed.
- A. VIttadini and M. Casarin, Theor. Chem. Acc., 2008, 120, 551–556 CrossRef CAS PubMed.
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183–191 CrossRef CAS PubMed.
- G. Rahman, V. M. García-Suárez and S. C. Hong, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 78, 184404 CrossRef.
- A. K. Singh, A. Janotti, M. Scheffler and C. G. Van de Walle, Phys. Rev. Lett., 2008, 101, 055502 CrossRef.
- C. Zener, Phys. Rev., 1951, 82, 403 CrossRef CAS.
- P. G. de Gennes, Phys. Rev., 1960, 118, 141 CrossRef CAS.
- R. Janisch and N. A. Spaldin, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73, 035201 CrossRef.
- A. Chaparadza and S. B. Rananavare, Nanotechnology, 2010, 21, 035708 CrossRef PubMed.
- K. Sato, P. H. Dederichs and H. Katayama-Yoshida, Europhys. Lett., 2000, 61(403), 1019 Search PubMed.
| This journal is © The Royal Society of Chemistry 2014 |