Template-free synthesis of core–shell CeO2 nanospheres†
Anran Xie,
Jinxin Guo,
Wei Liu and
Yanzhao Yang*
Key Laboratory for Special Functional Aggregate Materials of Education Ministry, School of Chemistry and Chemical Engineering, Shandong University, No.27 Shanda nanlu, Jinan, 250100, P. R. China. E-mail: yzhyang@sdu.edu.cn; Fax: +86-531-88564464; Tel: +86-531-88362988
First published on 6th January 2014
Abstract
Uniform CeO2 asymmetric core–shell nanospheres were easily synthesized via a mild template-free hydrothermal method. The results show that aggregated solid spheres transform into core–shell spheres through an Ostwald ripening process.
In recent years, core–shell materials have attracted much attention because of their potential applications1–3 in photonic devices, drug delivery, materials encapsulation, ionic intercalation, surface functionalization, and as nanocatalysts, membrane nanoreactors, etc. Compared with heterogeneous core–shell structured materials, homogeneous core–shell structured materials are more difficult to synthesize and as a result have been less well studied. To date, several hydrothermal and solvothermal methods have been developed for the fabrication of such core–shell nanostructures. For example, B. Liu et al. reported the preparation of homogeneous core–shell ZnS and Co3O4 spheres via a hydrothermal process.4 Wen-Shou Wang et al. investigated the mechanism for the synthesis of homogeneous core–shell CdMoO4 at room temperature.5 Chang-Wen Guo et al. fabricated stable core–shell TiO2 via hydrothermal precipitation.6 Jiaguo Yu et al. synthesized sphere-in-shell WO3·1/3H2O super-structures under mild hydrothermal conditions. Based on a series of particle self-transformations,7 Yuanhui Zheng et al. prepared core–shell metastable γ-MnS semiconductor nanocrystals via a simple solvothermal method.8 Wei Cheng et al. developed a simple approach for the synthesis of core–shell magnetite (Fe3O4) spheres based on the hydrothermal treatment of FeCl3, citrate, polyacrylamide (PAM) and urea.9
Ceria nanoparticles have a wide range of applications in catalysis, fuel cells, solar cells, UV blocks, chemical mechanical polishing for microelectronics, as sorbents for H2S removal, and metallurgical and glass/ceramic applications.10–16 Although much attention has been focused on the preparation of nanostructured CeO2, to the best of our knowledge, less effort has been made towards the fabrication of CeO2 core–shell structures.
Herein, we report the fabrication of asymmetric core–shell CeO2 nanoparticles via a template-free hydrothermal approach using Ce(NO3)3·6H2O as the cerium source, urea (CO(NH2)2) as the precipitating agent, citric acid (C6H8O7·H2O) as the directing agent and n-butanol as co-solvent (for experimental details, see ESI†). The structure and morphology of the core–shell CeO2 nanospheres (NPs) were characterized using scanning electron microscopy (SEM), transmission electron microscopy (TEM) and X-ray powder diffraction (XRD) techniques. Our study indicates that oriented attachment followed by Ostwald ripening is responsible for the formation of core–shell CeO2 NPs. Preliminary catalytic testing demonstrates that these core–shell CeO2 NPs are promising catalysts for CO oxidation.
The XRD pattern of the as-prepared NPs (Fig. S1, ESI†) shows the diffraction peaks can be indexed as cubic CeO2 (JCPDS no. 34-0394).
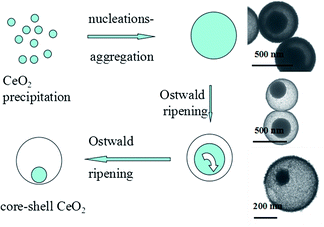
Fig. 1a and b show the morphology of CeO2 NPs by FE-SEM. From Fig. 1a it is obvious that the NPs were relatively monodisperse, with an average size of 500 nm. In Fig. 1b, the broken nanosphere has a core within it. The shell thickness is about 25 nm and the mean core diameter is about 150 nm.
The high resolution transmission electron microscopy (HRTEM) image of CeO2 NPs (Fig. S2, ESI†) shows the spacing between two adjacent lattice planes is 0.313 nm, which corresponds to the separation of the (200) lattice planes of CeO2. The sample is polycrystalline as indicated by the electron diffraction (ED) pattern (inset of Fig. S2†).
To investigate the formation and evolution of the homogeneous core–shell structure, time-dependent experiments were carried out. The synthetic process was ceased after different periods of precipitation: 4 h, 8 h, 12 h, 16 h, 20 h, 24 h. The TEM images (Fig. S3, ESI†) show the samples of different periods of precipitation. Fig. S3b† shows the sample treated for 8 h, which indicates the formation of solid spherical NPs. After treatment for 16 h, the core–shell structure began to appear through the hollow interior spaces between the cores and the shells. For longer reaction times, the hollow interior space expanded, due to the partial dissolution of cores. It can be observed that the outer part of the NPs was packed much more loosely than the inside, indicating different sequences of precipitation (the inside part was formed first). During the formation of the core–shell structure, the exterior appearance of the NPs did not change appreciably.
Based on the characterization data and the description of our experiments, we suggest that the whole hydrothermal synthetic process of the core–shell CeO2 NPs can be divided into three steps. Herein, in our reaction system, upon addition of H2O2 the solution changed from colorless to yellow. Thus it could be easily observed that Ce3+ was oxidized to form Ce4+. In the first stage after oxidation, cerium ions were precipitated in the form of cerium oxide. Via thermal decomposition, urea slowly released OH− ions. Cerium ions were then precipitated through combination with OH− ions. Citric acid acted as a directing agent by complexation of the three carboxyl groups with the cerium ions. In the second stage, these primary nuclei aggregated, driven by hydrophobic interactions and van der Waals attraction to minimize their surface energy, which means nucleation–aggregation. Therefore the interior part of the NPs was more densely accumulated compared with the exterior.
The Ostwald ripening process (which involves “the growth of larger crystals from those of smaller size which have a higher solubility than the larger ones”17,18) occurs during the third hydrothermal stage. The core of the NPs dissolves gradually, while the better crystallized parts on the surface of the NPs become denser, indicating that part of the core is recrystallized on the shell through a dissolution–redeposition process. Eventually, the core–shell CeO2 NPs is obtained (Fig. 2).
To demonstrate the potential application of ceria for oxygen storage/release, we studied the catalytic activity of the as-prepared products for CO oxidation. Compared with commercial CeO2, the as-prepared core–shell CeO2 NPs are more active at the same temperature (see Fig. 3). Xiguang Han et al.19 reported 81.0% CO conversion at 310 °C using hollow CeO2 cubes as a catalyst. In Chengsi Pan's research,20 the T50 of CeO2 nanowires synthesized at 110 °C for 24 h is 245 °C. In Zili Wu's work,21 the temperature for 20% CO conversion is 260 °C over ceria rods. For the core–shell CeO2, 20% CO conversion was achieved at 210 °C. At a temperature of 250 °C, we obtained 50% CO conversion, and 99% CO conversion was achieved at 317 °C. Previous reports10,22 revealed that better catalytic performance correlates well to nanocatalytic materials having higher surface areas. The BET specific surface area of core–shell CeO2 NPs is 55.0 m2 g−1 (Fig. S4, ESI†).
 | ||
| Fig. 3 CO conversion versus reaction temperature over (a) as-prepared core–shell CeO2 and (b) commercial CeO2 catalyst. | ||
Conclusions
In summary, we have developed a facile, mild and template-free hydrothermal strategy to synthesize core–shell CeO2 nanospheres with excellent catalytic performance for CO oxidation. Based on our study, the formation mechanism is proposed as nucleation–aggregation followed by Ostwald ripening during the hydrothermal reaction. For prolonged reaction times, the cores became smaller, therefore, we could control the hydrothermal reaction time to design and organize other nanospheres of specific core sizes using this method. In addition, the core–shell nanospheres are important in both theoretical investigations and technological applications.This work was supported by the Natural Science Foundation of China (grant nos 21076115 and 21276142).
Notes and references
- F. Caruso, R. A. Caruso and H. Mohwald, Science, 1998, 282, 1111 CrossRef CAS.
- Y. D. Yin, R. M. Rioux, C. K. Erdonmez, S. Hughes, G. A. Somorjai and A. P. Alivisatos, Science, 2004, 304, 711 CrossRef CAS PubMed.
- H. C. Zeng, J. Mater. Chem., 2006, 16, 649 RSC.
- B. Liu and H. C. Zeng, Small, 2005, 1, 266 Search PubMed.
- W.-S. Wang, L. Zhen, C.-Y. Xu and W.-Z. Shao, Cryst. Growth Des., 2009, 9, 1558 CAS.
- C.-W. Guo, Y. Cao, S.-H. Xie, W.-L. Dai and K.-N. Fan, Chem. Commun., 2003, 6, 700 RSC.
- J. Yu, H. Yu, H. Guo, M. Li and S. Mann, Small, 2008, 4, 87 CrossRef CAS PubMed.
- Y. Zheng, Y. Cheng, Y. Wang, L. Zhou, F. Bao and C. Jia, J. Phys. Chem. B, 2006, 110, 8284 CrossRef CAS PubMed.
- W. Cheng, K. Tang, Y. Qi, J. Sheng and Z. Liu, J. Mater. Chem., 2010, 20, 1799 RSC.
- K. B. Zhou, X. Wang, X. M. Sun, Q. Peng and Y. D. Li, J. Catal., 2005, 229, 206–212 CrossRef CAS PubMed.
- P. Dutta, S. Pal, M. S. Seehra, Y. Shi, E. M. Eyring and R. D. Ernst, Chem. Mater., 2006, 18, 5144–5146 CrossRef CAS.
- S. Tsunekawa, T. Fukuda and A. J. Kasuya, Appl. Phys., 2000, 87, 1318–1321 CAS.
- R. Yu, L. Yan, P. Zheng, J. Chen and X. Xing, J. Phys. Chem. C, 2008, 112, 19896 CAS.
- S. C. Laha and R. Ryoo, Chem. Commun., 2003, 17, 2138 RSC.
- R. DiMonte and J. Kaspar, J. Mater. Chem., 2005, 15, 633 RSC.
- B. Tang, L. Zhuo, J. Ge, G. Wang, Z. Shi and J. Niu, Chem. Commun., 2005, 28, 3565 RSC.
- W. Z. Ostwald, Phys. Chem., 1897, 22, 289 CAS.
- W. Z. Ostwald, Phys. Chem., 1990, 34, 495 Search PubMed.
- X. Han, L. Li and C. Wang, Nanoscale, 2013, 5, 7193–7196 RSC.
- C. Pan, D. Zhang, L. Shi and J. Fang, Eur. J. Inorg. Chem., 2008, 15, 2429–2436 CrossRef.
- Z. Wu, M. Li and S. H. Overbury, J. Catal., 2012, 285, 61–73 CrossRef CAS PubMed.
- P. K. Stoimenov, V. Zaikovski and K. J. Klabunde, J. Am. Chem. Soc., 2003, 125, 12907–12913 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra46292g |
| This journal is © The Royal Society of Chemistry 2014 |