Molecular dynamics simulations and microscopic analysis of the damping performance of hindered phenol AO-60/nitrile-butadiene rubber composites
Abstract
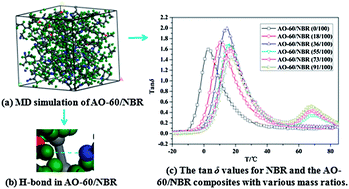
Molecular dynamics (MD) simulations are used to investigate the fundamental damping mechanism of the AO-60/nitrile-butadiene rubber (AO-60/NBR) composites at the molecular level in this study. The hydrogen bonds (H-bonds), binding energy, and fractional free volume (FFV) of the AO-60/NBR composites were obtained. The AO-60/NBR composite with an AO-60 content of 36 phr had the largest H-bonds, highest binding energy, and smallest FFV, all indicating a good compatibility between NBR and AO-60 and good damping performance of the AO-60/NBR composite. The experimental FTIR, and 1H-NMR results also showed that two types of H-bonds exist between the AO-60 small molecules and NBR polymer chains. Moreover, DSC and DMA were employed to characterize the compatibility between NBR and AO-60 in the composites. Phase separation between NBR and AO-60 appeared as the AO-60 content exceeded 36 phr. We hope the present study provides theoretical guidance for the design of optimum damping properties of polymer composites.

 Please wait while we load your content...
Please wait while we load your content...