A highly efficient one-pot method for the synthesis of thioureas and 2-imino-4-thiazolidinones under microwave conditions†
Abstract
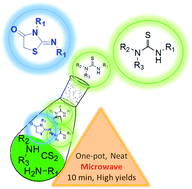
A one-pot synthesis of symmetrical and unsymmetrical substituted thioureas and 2-imino-4-thiazolidinones from simple starting materials under microwave irradiation and solventless conditions without base additives is presented. Various di- and trisubstituted thioureas are obtained in good yields in a few minutes. The sequential, three-component, one-pot synthesis of 2-imino-4-thiazolidinone derivatives is also studied, with satisfactory results obtained.

 Please wait while we load your content...
Please wait while we load your content...