Preparation of CeO2 nanoparticles supported on 1-D silica nanostructures for room temperature selective oxidation of styrene†
Abstract
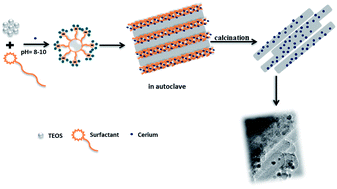
CeO2 nanoparticles of 2–5 nm size supported on 1-D silica nanostructure with diameter of ∼25–40 nm and a length of ∼1–4 μm were synthesized hydrothermally and it was found that the catalyst is very active for selective oxidation of styrene to styrene oxide at room temperature.

 Please wait while we load your content...
Please wait while we load your content...